Manipulation of components of the renin angiotensin system in renal proximal tubules fails to alter atherosclerosis in hypercholesterolemic mice
- PMID: 37655218
- PMCID: PMC10466789
- DOI: 10.3389/fcvm.2023.1250234
Manipulation of components of the renin angiotensin system in renal proximal tubules fails to alter atherosclerosis in hypercholesterolemic mice
Abstract
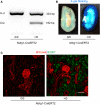
Background and objective: Whole body manipulation of the renin-angiotensin system (RAS) consistently exerts profound effects on experimental atherosclerosis development. A deficit in the literature has been a lack of attention to the effects of sex. Also, based on data with gene-deleted mice, the site of RAS activity that influences lesion formation is at an unknown distant location. Since angiotensin (AngII) concentrations are high in kidney and the major components of the RAS are present in renal proximal tubule cells (PTCs), this study evaluated the role of the RAS in PTCs in atherosclerosis development.
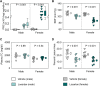
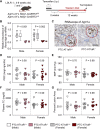
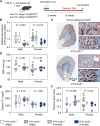
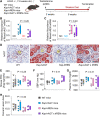
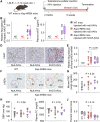
Methods and results: Mice with an LDL receptor -/- background were fed Western diet to induce hypercholesterolemia and atherosclerosis. We first demonstrated the role of AT1 receptor antagonism on atherosclerosis in both sexes. Losartan, an AngII type 1 (AT1) receptor blocker, had greater blood pressure-lowering effects in females than males, but equivalent effects between sexes in reducing atherosclerotic lesion size. To determine the roles of renal AT1a receptor and angiotensin-converting enzyme (ACE), either component was deleted in PTCs after weaning using a tamoxifen-inducible Cre expressed under the control of an Ndrg1 promoter. Despite profound deletion of AT1a receptor or ACE in PTCs, the absence of either protein did not influence development of atherosclerosis in either sex. Conversely, mice expressing human angiotensinogen and renin in PTCs or expressing human angiotensinogen in liver but human renin in PTCs did not change atherosclerotic lesion size in male mice.
Conclusion: Whole-body AT1R inhibition reduced atherosclerosis equivalently in both male and female mice; however, PTC-specific manipulation of the RAS components had no effects on hypercholesterolemia-induced atherosclerosis.
Keywords: AT1 receptor; angiotensin; angiotensin-converting enzyme; angiotensinogen; atherosclerosis; kidney; proximal tubules; renin.
© 2023 Kukida, Amioka, Ye, Chen, Moorleghen, Liang, Howatt, Katsumata, Yanagita, Sawada, Daugherty and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor.Circulation. 2004 Dec 21;110(25):3849-57. doi: 10.1161/01.CIR.0000150540.54220.C4. Epub 2004 Dec 13. Circulation. 2004. PMID: 15596561
-
Angiotensinogen and Megalin Interactions Contribute to Atherosclerosis-Brief Report.Arterioscler Thromb Vasc Biol. 2019 Feb;39(2):150-155. doi: 10.1161/ATVBAHA.118.311817. Arterioscler Thromb Vasc Biol. 2019. PMID: 30567480 Free PMC article.
-
Comparative effects of different modes of renin angiotensin system inhibition on hypercholesterolaemia-induced atherosclerosis.Br J Pharmacol. 2012 Mar;165(6):2000-2008. doi: 10.1111/j.1476-5381.2011.01712.x. Br J Pharmacol. 2012. PMID: 22014125 Free PMC article.
-
Recent Updates on the Proximal Tubule Renin-Angiotensin System in Angiotensin II-Dependent Hypertension.Curr Hypertens Rep. 2016 Aug;18(8):63. doi: 10.1007/s11906-016-0668-z. Curr Hypertens Rep. 2016. PMID: 27372447 Free PMC article. Review.
-
Megalin-Mediated Endocytosis in the Kidney Proximal Tubule: Relevance to Regulation of the Renal Renin-Angiotensin System.Nephron. 2023;147(3-4):244-249. doi: 10.1159/000526369. Epub 2022 Sep 12. Nephron. 2023. PMID: 36096093 Review.
Cited by
-
Renal Proximal Tubule Cell-Specific Megalin Deletion Does Not Affect Atherosclerosis But Induces Tubulointerstitial Nephritis in Mice Fed a Western Diet.Arterioscler Thromb Vasc Biol. 2025 Jan;45(1):74-89. doi: 10.1161/ATVBAHA.124.321366. Epub 2024 Nov 21. Arterioscler Thromb Vasc Biol. 2025. PMID: 39569521
-
Renal Proximal Tubule Cell-specific Megalin Deletion Does Not Affect Atherosclerosis But Induces Tubulointerstitial Nephritis in Mice Fed Western Diet.bioRxiv [Preprint]. 2024 Sep 27:2024.05.11.592234. doi: 10.1101/2024.05.11.592234. bioRxiv. 2024. Update in: Arterioscler Thromb Vasc Biol. 2025 Jan;45(1):74-89. doi: 10.1161/ATVBAHA.124.321366. PMID: 38798535 Free PMC article. Updated. Preprint.
-
Angiotensinogen as a Therapeutic Target for Cardiovascular and Metabolic Diseases.Arterioscler Thromb Vasc Biol. 2024 May;44(5):1021-1030. doi: 10.1161/ATVBAHA.124.318374. Epub 2024 Apr 4. Arterioscler Thromb Vasc Biol. 2024. PMID: 38572647 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

