Pharmacological diversity amongst approved and emerging antiseizure medications for the treatment of developmental and epileptic encephalopathies
- PMID: 37655228
- PMCID: PMC10467199
- DOI: 10.1177/17562864231191000
Pharmacological diversity amongst approved and emerging antiseizure medications for the treatment of developmental and epileptic encephalopathies
Abstract
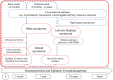
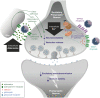
Developmental and epileptic encephalopathies (DEEs) are rare neurodevelopmental disorders characterised by early-onset and often intractable seizures and developmental delay/regression, and include Dravet syndrome and Lennox-Gastaut syndrome (LGS). Rufinamide, fenfluramine, stiripentol, cannabidiol and ganaxolone are antiseizure medications (ASMs) with diverse mechanisms of action that have been approved for treating specific DEEs. Rufinamide is thought to suppress neuronal hyperexcitability by preventing the functional recycling of voltage-gated sodium channels from the inactivated to resting state. It is licensed for adjunctive treatment of seizures associated with LGS. Fenfluramine increases extracellular serotonin levels and may reduce seizures via activation of specific serotonin receptors and positive modulation of the sigma-1 receptor. Fenfluramine is licensed for adjunctive treatment of seizures associated with Dravet syndrome and LGS. Stiripentol is a positive allosteric modulator of type-A gamma-aminobutyric acid (GABAA) receptors. As a broad-spectrum inhibitor of cytochrome P450 enzymes, its antiseizure effects may additionally arise through pharmacokinetic interactions with co-administered ASMs. Stiripentol is licensed for treating seizures associated with Dravet syndrome in patients taking clobazam and/or valproate. The mechanism(s) of action of cannabidiol remains largely unclear although multiple targets have been proposed, including transient receptor potential vanilloid 1, G protein-coupled receptor 55 and equilibrative nucleoside transporter 1. Cannabidiol is licensed as adjunctive treatment in conjunction with clobazam for seizures associated with Dravet syndrome and LGS, and as adjunctive treatment of seizures associated with tuberous sclerosis complex. Like stiripentol, ganaxolone is a positive allosteric modulator at GABAA receptors. It has recently been licensed in the USA for the treatment of seizures associated with cyclin-dependent kinase-like 5 deficiency disorder. Greater understanding of the causes of DEEs has driven research into the potential use of other novel and repurposed agents. Putative ASMs currently in clinical development for use in DEEs include soticlestat, carisbamate, verapamil, radiprodil, clemizole and lorcaserin.
Keywords: antiseizure medication; cannabidiol; developmental and epileptic encephalopathies; epilepsy; fenfluramine; ganaxolone; rufinamide; stiripentol.
© The Author(s), 2023.
Conflict of interest statement
Over the past 36 months, Dr Sills has received consultancy and/or speaker fees for projects and presentations related to the experimental and clinical pharmacology of antiseizure drugs from Angelini Pharma, Arvelle Therapeutics GmbH, Bial Pharma UK Ltd, Desitin Pharma Ltd, Eisai Ltd, UCB Pharma Ltd and Zogenix International Ltd.
Figures

References
-
- Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010; 51: 676–685. - PubMed
-
- Scheffer IE, Liao J. Deciphering the concepts behind “Epileptic encephalopathy” and “Developmental and epileptic encephalopathy. Eur J Paediatr Neurol 2020; 24: 11–14. - PubMed
-
- Lin JJ, Meletti S, Vaudano AE, et al. Developmental and epileptic encephalopathies: is prognosis related to different epileptic network dysfunctions? Epilepsy Behav 2022; 131: 107654. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

