3D-Printed Osteoinductive Polymeric Scaffolds with Optimized Architecture to Repair a Sheep Metatarsal Critical-Size Bone Defect
- PMID: 37655491
- PMCID: PMC11468956
- DOI: 10.1002/adhm.202301692
3D-Printed Osteoinductive Polymeric Scaffolds with Optimized Architecture to Repair a Sheep Metatarsal Critical-Size Bone Defect
Abstract
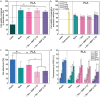
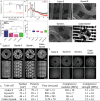
The reconstruction of critical-size bone defects in long bones remains a challenge for clinicians. A new osteoinductive medical device is developed here for long bone repair by combining a 3D-printed architectured cylindrical scaffold made of clinical-grade polylactic acid (PLA) with a polyelectrolyte film coating delivering the osteogenic bone morphogenetic protein 2 (BMP-2). This film-coated scaffold is used to repair a sheep metatarsal 25-mm long critical-size bone defect. In vitro and in vivo biocompatibility of the film-coated PLA material is proved according to ISO standards. Scaffold geometry is found to influence BMP-2 incorporation. Bone regeneration is followed using X-ray scans, µCT scans, and histology. It is shown that scaffold internal geometry, notably pore shape, influenced bone regeneration, which is homogenous longitudinally. Scaffolds with cubic pores of ≈870 µm and a low BMP-2 dose of ≈120 µg cm-3 induce the best bone regeneration without any adverse effects. The visual score given by clinicians during animal follow-up is found to be an easy way to predict bone regeneration. This work opens perspectives for a clinical application in personalized bone regeneration.
Keywords: 3D printing; bone tissue engineering; large animal models; medical devices; surface coatings.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Decambron A., Fournet A., Bensidhoum M., Manassero M., Sailhan F., Petite H., Logeart‐Avramoglou D., Viateau V., J. Orthop. Res. 2017, 35, 2637. - PubMed
-
- Norris B. L., Vanderkarr M., Sparks C., Chitnis A. S., Ray B., Holy C. E., Injury 2021, 52, 2935. - PubMed
-
- Pape H. C., Evans A., Kobbe P., J. Orthop. Trauma 2010, 24, S36. - PubMed
-
- Laurencin C., Khan Y., El‐Amin S. F., Expert Rev. Med. Devices 2006, 3, 49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

