The crucial role of silver(I)-salts as additives in C-H activation reactions: overall analysis of their versatility and applicability
- PMID: 37655711
- PMCID: PMC10714919
- DOI: 10.1039/d3cs00328k
The crucial role of silver(I)-salts as additives in C-H activation reactions: overall analysis of their versatility and applicability
Abstract
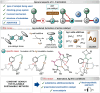
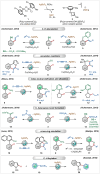
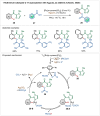
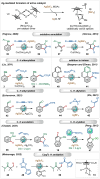
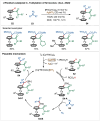
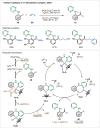
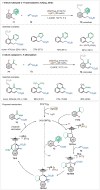
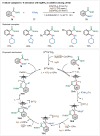
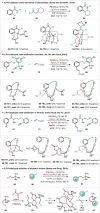
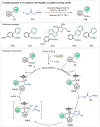
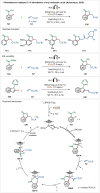
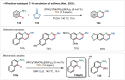
Transition-metal catalyzed C-H activation reactions have been proven to be useful methodologies for the assembly of synthetically meaningful molecules. This approach bears intrinsic peculiarities that are important to be studied and comprehended in order to achieve its best performance. One example is the use of additives for the in situ generation of catalytically active species. This strategy varies according to the type of additive and the nature of the pre-catalyst that is being used. Thus, silver(I)-salts have proven to play an important role, due to the resulting high reactivity derived from the pre-catalysts of the main transition metals used so far. While being powerful and versatile, the use of silver-based additives can raise concerns, since superstoichiometric amounts of silver(I)-salts are typically required. Therefore, it is crucial to first understand the role of silver(I) salts as additives, in order to wisely overcome this barrier and shift towards silver-free systems.
Conflict of interest statement
There are no conflicts to declare.
Figures













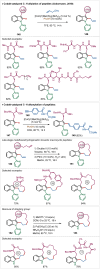
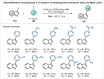






Similar articles
-
Silver-Catalyzed Activation of Terminal Alkynes for Synthesizing Nitrogen-Containing Molecules.Acc Chem Res. 2020 Mar 17;53(3):662-675. doi: 10.1021/acs.accounts.9b00623. Epub 2020 Feb 20. Acc Chem Res. 2020. PMID: 32078302
-
Silver-Free C-H Activation: Strategic Approaches towards Realizing the Full Potential of C-H Activation in Sustainable Organic Synthesis.Angew Chem Int Ed Engl. 2022 Nov 25;61(48):e202210825. doi: 10.1002/anie.202210825. Epub 2022 Oct 21. Angew Chem Int Ed Engl. 2022. PMID: 36062882 Free PMC article. Review.
-
Is silver a mere terminal oxidant in palladium catalyzed C-H bond activation reactions?Chem Sci. 2019 Nov 13;11(1):208-216. doi: 10.1039/c9sc04540f. eCollection 2020 Jan 7. Chem Sci. 2019. PMID: 32110372 Free PMC article.
-
Copper, silver, and gold complexes in hydrosilylation reactions.Acc Chem Res. 2008 Feb;41(2):349-58. doi: 10.1021/ar7001655. Acc Chem Res. 2008. PMID: 18281951
-
Recent Strategies in Transition-Metal-Catalyzed Sequential C-H Activation/Annulation for One-Step Construction of Functionalized Indazole Derivatives.Molecules. 2022 Aug 3;27(15):4942. doi: 10.3390/molecules27154942. Molecules. 2022. PMID: 35956893 Free PMC article. Review.
Cited by
-
Enantioselective β-C(sp3)-H Nucleophilic Tosylation of Native Amides: A Synthetic Platform for Chiral Methyl Stereocenters.J Am Chem Soc. 2025 Jun 11;147(23):19559-19567. doi: 10.1021/jacs.4c17267. Epub 2025 May 31. J Am Chem Soc. 2025. PMID: 40448573 Free PMC article.
-
Crafting 1,4-diaryl spirobifluorene hosts in OLEDs via interannular C-H arylation: synergistic effects of molecular linearity and orthogonality.Chem Sci. 2024 Jun 4;15(27):10547-10555. doi: 10.1039/d4sc02178a. eCollection 2024 Jul 10. Chem Sci. 2024. PMID: 38994415 Free PMC article.
-
Platform Design Enabling Silver(III) Stabilization ─ The Uprise of AgIIICF3 Chemistry?Chemistry. 2025 Aug 7;31(44):e202501606. doi: 10.1002/chem.202501606. Epub 2025 Jul 21. Chemistry. 2025. PMID: 40685896 Free PMC article. Review.
-
Pd-Catalyzed C(sp2)-H/C(sp2)-H Coupling of Limonene.J Org Chem. 2024 Aug 2;89(15):10451-10461. doi: 10.1021/acs.joc.4c00501. Epub 2024 Jul 18. J Org Chem. 2024. PMID: 39025478 Free PMC article.
-
Palladium-Catalyzed Oxidative Cyclization of O-Aryl Cyclic Vinylogous Esters: Synthesis of Benzofuran-Fused Cyclohexenones.J Org Chem. 2024 Dec 20;89(24):18679-18683. doi: 10.1021/acs.joc.4c02167. Epub 2024 Nov 28. J Org Chem. 2024. PMID: 39610223 Free PMC article.
References
-
- Rogge T. Kaplaneris N. Chatani N. Kim J. Chang S. Punji B. Schafer L. L. Musaev D. G. Wencel-Delord J. Roberts C. A. Sarpong R. Wilson Z. E. Brimble M. A. Johansson M. J. Ackermann L. Nature Rev. Meth. Primers. 2021;1:43. doi: 10.1038/s43586-021-00041-2. - DOI
- Yoshino T. Matsunaga S. ACS Catal. 2021;11:6455–6466. doi: 10.1021/acscatal.1c01351. - DOI
- Gandeepan P. Finger L. H. Meyer T. H. Ackermann L. Chem. Soc. Rev. 2020;49:4254–4272. doi: 10.1039/D0CS00149J. - DOI - PubMed
- Li X. Ouyang W. Nie J. Ji S. Chen Q. Huo Y. ChemCatChem. 2020;12:2358–2384. doi: 10.1002/cctc.201902150. - DOI
- Gandeepan P. Müller T. Zell D. Cera G. Warratz S. Ackermann L. Chem. Rev. 2019;119:2192–2452. doi: 10.1021/acs.chemrev.8b00507. - DOI - PubMed
- Liu W. Ackermann L. ACS Catal. 2016;6:3743–3752. doi: 10.1021/acscatal.6b00993. - DOI
- Li S. S. Qin L. Dong L. Org. Biomol. Chem. 2016;14:4554–4570. doi: 10.1039/C6OB00209A. - DOI - PubMed
- Khan F. F. Sinha S. K. Lahiri G. K. Maiti D. Chem. Asian J. 2018;13:2243–2256. doi: 10.1002/asia.201800545. - DOI - PubMed
- Moselage M. Li J. Ackermann L. ACS Catal. 2016;6:498–525. doi: 10.1021/acscatal.5b02344. - DOI
- Ackermann L. Acc. Chem. Res. 2014;47:281–295. doi: 10.1021/ar3002798. - DOI - PubMed
- De Sarkar S. Liu W. Kozhushkov S. I. Ackermann L. Adv. Synth. Catal. 2014;356:1461–1479. doi: 10.1002/adsc.201400110. - DOI
- Ackermann L. Acc. Chem. Res. 2014;47:281–295. doi: 10.1021/ar3002798. - DOI - PubMed
- Kozhushkov S. I. Ackermann L. Chem. Sci. 2013;4:886–896. doi: 10.1039/C2SC21524A. - DOI
- Kuhl N. Hopkinson M. N. Wencel-Delord J. Glorius F. Angew. Chem., Int. Ed. 2012;51:10236–10254. doi: 10.1002/anie.201203269. - DOI - PubMed
- Arockiam P. B. Bruneau C. Dixneuf P. H. Chem. Rev. 2012;112:5879–5918. doi: 10.1021/cr300153j. - DOI - PubMed
- Ackermann L. Chem. Rev. 2011;111:1315–1345. doi: 10.1021/cr100412j. - DOI - PubMed
- Deb A. Manna S. Modak A. Patra T. Maity S. Maiti D. Angew. Chem., Int. Ed. 2013;52:9747–9750. doi: 10.1002/anie.201303576. - DOI - PubMed
- Maity S. Manna S. Rana S. Naveen T. Mallick A. Maiti D. J. Am. Chem. Soc. 2013;135:3355–3358. doi: 10.1021/ja311942e. - DOI - PubMed
- Maji A. Hazra A. Maiti D. Org. Lett. 2014;16:4524–4527. doi: 10.1021/ol502071g. - DOI - PubMed
-
- Guillemard L. Kaplaneris N. Ackermann L. Johansson M. J. Nat. Rev. Chem. 2021;5:522–545. doi: 10.1038/s41570-021-00300-6. - DOI - PubMed
- de Carvalho R. L. de Miranda A. S. Nunes M. P. Gomes R. S. Jardim G. A. M. da Silva Júnior E. N. Beilstein J. Org. Chem. 2021;17:1849–1938. doi: 10.3762/bjoc.17.126. - DOI - PMC - PubMed
- Dey A. Maity S. Maiti S. Chem. Commun. 2016;52:12398–12414. doi: 10.1039/C6CC05235E. - DOI - PubMed
- Song G. Wang F. Li X. Chem. Soc. Rev. 2012;41:3651–3678. doi: 10.1039/C2CS15281A. - DOI - PubMed
-
- Murali K. Machado L. A. de Carvalho R. L. Pedrosa L. F. Mukherjee R. da Silva Júnior E. N. Maiti D. Chem. – Eur. J. 2021;27:12453–12508. doi: 10.1002/chem.202101004. - DOI - PubMed
- de Carvalho R. L. Almeida R. G. Murali K. Machado L. A. Pedrosa L. F. Dolui P. Maiti D. da Silva Júnior E. N. Org. Biomol. Chem. 2021;19:525–547. doi: 10.1039/D0OB02232B. - DOI - PubMed
-
- Xue X.-S. Ji P. Zhou B. Cheng J.-P. Chem. Rev. 2017;117:8622–8648. doi: 10.1021/acs.chemrev.6b00664. - DOI - PubMed
- Hummel J. R. Boerth J. A. Ellman J. A. Chem. Rev. 2017;117:9163–9227. doi: 10.1021/acs.chemrev.6b00661. - DOI - PMC - PubMed
- He J. Wasa M. Chan K. S. L. Shao Q. Yu J.-Q. Chem. Rev. 2017;117:8754–8786. doi: 10.1021/acs.chemrev.6b00622. - DOI - PMC - PubMed
- Piou T. Rovis T. Acc. Chem. Res. 2018;51:170–180. doi: 10.1021/acs.accounts.7b00444. - DOI - PMC - PubMed
- Vásquez-Céspedes S. Wang X. Glorius F. ACS Catal. 2018;8:242–257. doi: 10.1021/acscatal.7b03048. - DOI
- Lu M.-Z. Goh J. Maraswami M. Jia Z. Tian J.-S. Loh T.-P. Chem. Rev. 2022;122:17479–17646. doi: 10.1021/acs.chemrev.2c00032. - DOI - PubMed
- Rej S. Ano Y. Chatani N. Chem. Rev. 2020;120:1788–1887. doi: 10.1021/acs.chemrev.9b00495. - DOI - PubMed
- Dey A. Sinha S. K. Achar T. K. Maiti D. Angew. Chem., Int. Ed. 2018;58:10820–10843. doi: 10.1002/anie.201812116. - DOI - PubMed
- Chen Z. Wang B. Zhang J. Yu W. Liu Z. Zhang Y. Org. Chem. Front. 2015;2:1107–1295. doi: 10.1039/C5QO00004A. - DOI
- Zhu R.-Y. Farmer M. E. Chen Y.-Q. Yu J.-Q. Angew. Chem., Int. Ed. 2016;55:10578–10599. doi: 10.1002/anie.201600791. - DOI - PMC - PubMed
- Zakis J. M. Smejkal T. Wencel-Delord J. Chem. Commun. 2022;58:483–490. doi: 10.1039/D1CC05195D. - DOI - PubMed
- Haldar C. Hoque M. E. Bisht R. Chattopadhyay B. Tetrahedron Lett. 2018;59:1269–1277. doi: 10.1016/j.tetlet.2018.01.098. - DOI
- Baudoin O. Acc. Chem. Res. 2017;50:1114–1123. doi: 10.1021/acs.accounts.7b00099. - DOI - PubMed
- Minami Y. Hiyama T. Acc. Chem. Res. 2016;49:67–77. doi: 10.1021/acs.accounts.5b00414. - DOI - PubMed
- Woźniak Ł. Cramer N. Trends. Chem. 2019;1:471–484. doi: 10.1016/j.trechm.2019.03.013. - DOI
- Ackermann L. Acc. Chem. Res. 2020;53:84–104. doi: 10.1021/acs.accounts.9b00510. - DOI - PubMed
- Loup J. Dhawa U. Pesciaioli F. Wencel-Delord J. Ackermann L. Angew. Chem., Int. Ed. 2019;58:12803–12818. doi: 10.1002/anie.201904214. - DOI - PubMed
-
- Ghosh S. Shilpa S. Athira C. Sunoj R. B. Topics in Catal. 2022;65:141–164. doi: 10.1007/s11244-021-01527-9. - DOI
- Bhattacharya T. Dutta S. Maiti D. ACS Catal. 2021;11:9702–9714. doi: 10.1021/acscatal.1c02552. - DOI
- Mudarra Á. L. Martínez De Salinas S. Pérez-Temprano M. H. Org. Biomol. Chem. 2019;17:1655–1667. doi: 10.1039/C8OB02611D. - DOI - PubMed
- Bay K. L. Yang Y.-F. Houk K. N. J. Organomet. Catal. 2018;864:19–25. doi: 10.1016/j.jorganchem.2017.12.026. - DOI
Publication types
LinkOut - more resources
Full Text Sources

