Unveiling the Crucial Roles of O2•- and ATP in Hepatic Ischemia-Reperfusion Injury Using Dual-Color/Reversible Fluorescence Imaging
- PMID: 37655757
- PMCID: PMC10510312
- DOI: 10.1021/jacs.3c04303
Unveiling the Crucial Roles of O2•- and ATP in Hepatic Ischemia-Reperfusion Injury Using Dual-Color/Reversible Fluorescence Imaging
Abstract
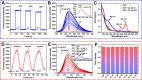
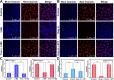
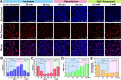
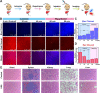
Hepatic ischemia-reperfusion injury (HIRI) is mainly responsible for morbidity or death due to graft rejection after liver transplantation. During HIRI, superoxide anion (O2•-) and adenosine-5'-triphosphate (ATP) have been identified as pivotal biomarkers associated with oxidative stress and energy metabolism, respectively. However, how the temporal and spatial fluctuations of O2•- and ATP coordinate changes in HIRI and particularly how they synergistically regulate each other in the pathological mechanism of HIRI remains unclear. Herein, we rationally designed and successfully synthesized a dual-color and dual-reversible molecular fluorescent probe (UDP) for dynamic and simultaneous visualization of O2•- and ATP in real-time, and uncovered their interrelationship and synergy in HIRI. UDP featured excellent sensitivity, selectivity, and reversibility in response to O2•- and ATP, which rendered UDP suitable for detecting O2•- and ATP and generating independent responses in the blue and red fluorescence channels without spectral crosstalk. Notably, in situ imaging with UDP revealed for the first time synchronous O2•- bursts and ATP depletion in hepatocytes and mouse livers during the process of HIRI. Surprisingly, a slight increase in ATP was observed during reperfusion. More importantly, intracellular O2•-─succinate dehydrogenase (SDH)─mitochondrial (Mito) reduced nicotinamide adenine dinucleotide (NADH)─Mito ATP─intracellular ATP cascade signaling pathway in the HIRI process was unveiled which illustrated the correlation between O2•- and ATP for the first time. This research confirms the potential of UDP for the dynamic monitoring of HIRI and provides a clear illustration of HIRI pathogenesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Reversible Fluorescent Probes for Dynamic Imaging of Liver Ischemia-Reperfusion Injury.Acc Chem Res. 2024 Sep 3;57(17):2594-2605. doi: 10.1021/acs.accounts.4c00449. Epub 2024 Aug 20. Acc Chem Res. 2024. PMID: 39164205 Review.
-
Uncovering Endoplasmic Reticulum Superoxide Regulating Hepatic Ischemia-Reperfusion Injury by Dynamic Reversible Fluorescence Imaging.Anal Chem. 2023 May 30;95(21):8367-8375. doi: 10.1021/acs.analchem.3c01068. Epub 2023 May 18. Anal Chem. 2023. PMID: 37200499
-
Dual-color reversible fluorescent carbon dots designed for dynamic monitoring of cellular superoxide anion radicals.J Mater Chem B. 2025 May 1;13(17):5163-5170. doi: 10.1039/d5tb00099h. J Mater Chem B. 2025. PMID: 40205991
-
In situ and real-time imaging of superoxide anion and peroxynitrite elucidating arginase 1 nitration aggravating hepatic ischemia-reperfusion injury.Biomaterials. 2019 Dec;225:119499. doi: 10.1016/j.biomaterials.2019.119499. Epub 2019 Sep 16. Biomaterials. 2019. PMID: 31561087
-
Research progress of lncRNA and miRNA in hepatic ischemia-reperfusion injury.Hepatobiliary Pancreat Dis Int. 2023 Feb;22(1):45-53. doi: 10.1016/j.hbpd.2022.07.008. Epub 2022 Jul 30. Hepatobiliary Pancreat Dis Int. 2023. PMID: 35934611 Review.
Cited by
-
Involvement of intestinal mucosal microbiota in adenine-induced liver function injury.3 Biotech. 2025 Jan;15(1):6. doi: 10.1007/s13205-024-04180-7. Epub 2024 Dec 12. 3 Biotech. 2025. PMID: 39676888
-
Photoactivated hydride therapy under hypoxia beyond ROS.Chem Sci. 2024 Nov 12;15(48):20292-20302. doi: 10.1039/d4sc06576j. eCollection 2024 Dec 11. Chem Sci. 2024. PMID: 39568933 Free PMC article.
-
Risk factors and predictive models for postoperative surgical site infection in patients with massive hemorrhage.J Orthop. 2024 Aug 11;69:61-67. doi: 10.1016/j.jor.2024.08.005. eCollection 2025 Nov. J Orthop. 2024. PMID: 40183036
-
Multifunctional Fluorescent Probe for Simultaneous Detection of ATP, Cys, Hcy, and GSH: Advancing Insights into Epilepsy and Liver Injury.Adv Sci (Weinh). 2025 Mar;12(11):e2415882. doi: 10.1002/advs.202415882. Epub 2025 Jan 30. Adv Sci (Weinh). 2025. PMID: 39887673 Free PMC article.
-
A versatile NIR probe for multifunctional detection of tumors, fatty liver, and liver injury.Chem Sci. 2025 Jun 12;16(27):12408-12415. doi: 10.1039/d5sc01433f. eCollection 2025 Jul 10. Chem Sci. 2025. PMID: 40521115 Free PMC article.
References
-
- Li J.; Li R.; Lv G.; Liu H. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2036–2047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

