Mitochondrial phospholipid metabolism in health and disease
- PMID: 37655851
- PMCID: PMC10482392
- DOI: 10.1242/jcs.260857
Mitochondrial phospholipid metabolism in health and disease
Abstract
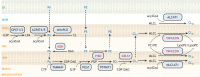
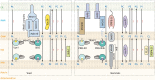
Studies of rare human genetic disorders of mitochondrial phospholipid metabolism have highlighted the crucial role that membrane phospholipids play in mitochondrial bioenergetics and human health. The phospholipid composition of mitochondrial membranes is highly conserved from yeast to humans, with each class of phospholipid performing a specific function in the assembly and activity of various mitochondrial membrane proteins, including the oxidative phosphorylation complexes. Recent studies have uncovered novel roles of cardiolipin and phosphatidylethanolamine, two crucial mitochondrial phospholipids, in organismal physiology. Studies on inter-organellar and intramitochondrial phospholipid transport have significantly advanced our understanding of the mechanisms that maintain mitochondrial phospholipid homeostasis. Here, we discuss these recent advances in the function and transport of mitochondrial phospholipids while describing their biochemical and biophysical properties and biosynthetic pathways. Additionally, we highlight the roles of mitochondrial phospholipids in human health by describing the various genetic diseases caused by disruptions in their biosynthesis and discuss advances in therapeutic strategies for Barth syndrome, the best-studied disorder of mitochondrial phospholipid metabolism.
Keywords: Barth syndrome; Cardiolipin; Membranes; Mitochondria; Phosphatidylethanolamine; Phospholipids.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Achleitner, G., Gaigg, B., Krasser, A., Kainersdorfer, E., Kohlwein, S. D., Perktold, A., Zellnig, G. and Daum, G. (1999). Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur. J. Biochem. 264, 545-553. 10.1046/j.1432-1327.1999.00658.x - DOI - PubMed
-
- Adachi, Y., Itoh, K., Yamada, T., Cerveny, K. L., Suzuki, T. L., Macdonald, P., Frohman, M. A., Ramachandran, R., Iijima, M. and Sesaki, H. (2016). Coincident phosphatidic acid interaction restrains Drp1 in mitochondrial division. Mol. Cell 63, 1034-1043. 10.1016/j.molcel.2016.08.013 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

