High selectivity of the hyperthermophilic subtilase propeptide domain toward inhibition of its cognate protease
- PMID: 37655909
- PMCID: PMC10580911
- DOI: 10.1128/spectrum.01487-23
High selectivity of the hyperthermophilic subtilase propeptide domain toward inhibition of its cognate protease
Abstract
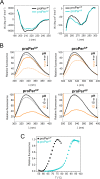
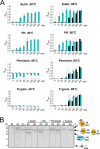
Microbial extracellular subtilases are highly active proteolytic enzymes commonly used in commercial applications. These subtilases are synthesized in their inactive proform, which matures into the active protease under the control of the propeptide domain. In mesophilic bacterial prosubtilases, the propeptide functions as both an obligatory chaperone and an inhibitor of the subtilase catalytic domain. In contrast, the propeptides of hyperthermophilic archaeal prosubtilases act mainly as tight inhibitors and are not essential for subtilase folding. It is unclear whether this stronger inhibitory activity of hyperthermophilic propeptides results in their higher selectivity toward their cognate subtilases, in contrast to promiscuous mesophilic propeptides. Here, we showed that the propeptide of pernisine, a hyperthermostable archaeal subtilase, strongly interacts with and inhibits pernisine, but not the homologous subtilisin Carlsberg and proteinase K. Instead, the pernisine propeptide was readily degraded by subtilisin Carlsberg and proteinase K. In addition, the catalytic domain of unprocessed propernisine was also susceptible to degradation but became proteolytically stable after autoprocessing of propernisine into the inactive, noncovalent complex propeptide:pernisine. This allowed efficient transactivation of the autoprocessed complex propeptide:pernisine through degradation of pernisine propeptide by subtilisin Carlsberg and proteinase K at mesophilic temperature. Moreover, we demonstrated that active pernisine molecules are inhibited by the propeptide that is released after pernisine-catalyzed degradation of the unprocessed propernisine catalytic domain. This highlights the high inhibitory potency of the hyperthermophilic propeptide toward its cognate subtilase and its importance in regulating subtilase maturation, to prevent the degradation of the unprocessed subtilase precursors by the prematurely activated molecules. IMPORTANCE Many microorganisms secrete proteases into their environment to degrade protein substrates for their growth. The important group of these extracellular enzymes are subtilases, which are also widely used in practical applications. These subtilases are inhibited by their propeptide domain, which is degraded during the prosubtilase maturation process. Here, we showed that the propeptide of pernisine, a prion-degrading subtilase from the hyperthermophilic archaeon, strongly inhibits pernisine with extraordinarily high binding affinity. This interaction proved to be highly selective, as pernisine propeptide was rapidly degraded by mesophilic pernisine homologs. This in turn allowed rapid transactivation of propernisine by mesophilic subtilases at lower temperatures, which might simplify the procedures for preparation of active pernisine for commercial use. The results reported in this study suggest that the hyperthermophilic subtilase propeptide evolved to function as tight and selective regulator of maturation of the associated prosubtilase to prevent its premature activation under high temperatures.
Keywords: enzyme activation; enzyme inhibition; hyperthermophile; propeptide; protein stability; subtilase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Insights into the Maturation of Pernisine, a Subtilisin-Like Protease from the Hyperthermophilic Archaeon Aeropyrum pernix.Appl Environ Microbiol. 2020 Aug 18;86(17):e00971-20. doi: 10.1128/AEM.00971-20. Print 2020 Aug 18. Appl Environ Microbiol. 2020. PMID: 32561587 Free PMC article.
-
Ca2+-dependent maturation of subtilisin from a hyperthermophilic archaeon, Thermococcus kodakaraensis: the propeptide is a potent inhibitor of the mature domain but is not required for its folding.Appl Environ Microbiol. 2006 Jun;72(6):4154-62. doi: 10.1128/AEM.02696-05. Appl Environ Microbiol. 2006. PMID: 16751527 Free PMC article.
-
A novel subtilase inhibitor in plants shows structural and functional similarities to protease propeptides.J Biol Chem. 2017 Apr 14;292(15):6389-6401. doi: 10.1074/jbc.M117.775445. Epub 2017 Feb 21. J Biol Chem. 2017. PMID: 28223360 Free PMC article.
-
Protease propeptide structures, mechanisms of activation, and functions.Crit Rev Biochem Mol Biol. 2020 Apr;55(2):111-165. doi: 10.1080/10409238.2020.1742090. Epub 2020 Apr 14. Crit Rev Biochem Mol Biol. 2020. PMID: 32290726 Review.
-
Insights from bacterial subtilases into the mechanisms of intramolecular chaperone-mediated activation of furin.Methods Mol Biol. 2011;768:59-106. doi: 10.1007/978-1-61779-204-5_4. Methods Mol Biol. 2011. PMID: 21805238 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

