In vivo efficacy of pitavastatin combined with itraconazole against Aspergillus fumigatus in silkworm models
- PMID: 37655910
- PMCID: PMC10581172
- DOI: 10.1128/spectrum.02666-23
In vivo efficacy of pitavastatin combined with itraconazole against Aspergillus fumigatus in silkworm models
Abstract
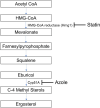
Azole resistance in Aspergillus fumigatus is a worldwide concern and new antifungal drugs are required to overcome this problem. Statin, a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, has been reported to suppress the growth of A. fumigatus, but little is known about its in vivo antifungal effect against A. fumigatus. In this study, we evaluated the in vivo efficacy of pitavastatin (PIT) combined with itraconazole (ITC) against azole-susceptible and azole-resistant strains with silkworm models. Prolongation of survival was confirmed in the combination-therapy (PIT and ITC) group compared to the no-treatment group in both azole-susceptible and azole-resistant strain models. Furthermore, when the azole-susceptible strain was used, the combination-therapy resulted in a higher survival rate than with ITC alone. Histopathological analysis of the silkworms revealed a reduction of the hyphal amount in both azole-susceptible and azole-resistant strain models. Quantitative evaluation of fungal DNA by qPCR in azole-susceptible strain models clarified the reduction of fungal burden in the combination-therapy group compared with the no-treatment group and ITC-alone group. These results indicate that the efficacy of PIT was enhanced when combined with ITC in vivo. As opposed to most statins, PIT has little drug-drug interaction with azoles in humans and can be used safely with ITC. This combination therapy may be a promising option as an effective treatment in clinical settings in the future. IMPORTANCE Azole resistance among A. fumigatus isolates has recently been increasingly recognized as a cause of treatment failure, and alternative antifungal therapies are required to overcome this problem. Our study shows the in vivo efficacy of PIT combined with ITC against A. fumigatus using silkworm models by several methods including evaluation of survival rates, histopathological analysis, and assessment of fungal burden. Contrary to most statins, PIT can be safely administered with azoles because of less drug-drug interactions, so this study should help us to verify how to make use of the drug in clinical settings in the future.
Keywords: Aspergillus fumigatus; azoles; checkerboard; histopathological analysis; in vivo efficacy; qPCR; silkworm; statin; survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
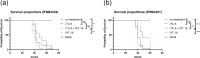


Similar articles
-
In Vitro and In Vivo Interactions of TOR Inhibitor AZD8055 and Azoles against Pathogenic Fungi.Microbiol Spectr. 2022 Feb 23;10(1):e0200721. doi: 10.1128/spectrum.02007-21. Epub 2022 Jan 12. Microbiol Spectr. 2022. PMID: 35019705 Free PMC article.
-
Synergistic Effect of Pyrvinium Pamoate and Azoles Against Aspergillus fumigatus in vitro and in vivo.Front Microbiol. 2020 Nov 3;11:579362. doi: 10.3389/fmicb.2020.579362. eCollection 2020. Front Microbiol. 2020. PMID: 33224118 Free PMC article.
-
High detection rate of azole-resistant Aspergillus fumigatus after treatment with azole antifungal drugs among patients with chronic pulmonary aspergillosis in a single hospital setting with low azole resistance.Med Mycol. 2021 Apr 6;59(4):327-334. doi: 10.1093/mmy/myaa052. Med Mycol. 2021. PMID: 32642756
-
Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment.Dan Med J. 2016 Oct;63(10):B5288. Dan Med J. 2016. PMID: 27697142 Review.
-
How to: EUCAST recommendations on the screening procedure E.Def 10.1 for the detection of azole resistance in Aspergillus fumigatus isolates using four-well azole-containing agar plates.Clin Microbiol Infect. 2019 Jun;25(6):681-687. doi: 10.1016/j.cmi.2018.09.008. Epub 2018 Sep 28. Clin Microbiol Infect. 2019. PMID: 30268672 Review.
Cited by
-
Synergistic Antifungal Activity of PIT and ITZ Against Varied Aspergillus Species via Affecting The Ergosterol Content and Intracellular Drug Retention.Curr Microbiol. 2025 Mar 17;82(5):198. doi: 10.1007/s00284-025-04150-z. Curr Microbiol. 2025. PMID: 40095083
-
Synergistic potential of lopinavir and azole combinational therapy against clinically important Aspergillus species.PLoS One. 2024 Dec 2;19(12):e0314474. doi: 10.1371/journal.pone.0314474. eCollection 2024. PLoS One. 2024. PMID: 39621587 Free PMC article.
References
-
- Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C, European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society . 2016. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 47:45–68. doi:10.1183/13993003.00583-2015 - DOI - PubMed
-
- Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young J-A, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis 63:433–442. doi:10.1093/cid/ciw444 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

