Maintenance of heme homeostasis in Staphylococcus aureus through post-translational regulation of glutamyl-tRNA reductase
- PMID: 37655914
- PMCID: PMC10521356
- DOI: 10.1128/jb.00171-23
Maintenance of heme homeostasis in Staphylococcus aureus through post-translational regulation of glutamyl-tRNA reductase
Abstract
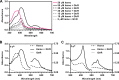
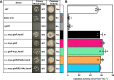
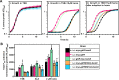
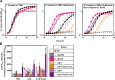
Staphylococcus aureus is an important human pathogen responsible for a variety of infections including skin and soft tissue infections, endocarditis, and sepsis. The combination of increasing antibiotic resistance in this pathogen and the lack of an efficacious vaccine underscores the importance of understanding how S. aureus maintains metabolic homeostasis in a variety of environments, particularly during infection. Within the host, S. aureus must regulate cellular levels of the cofactor heme to support enzymatic activities without encountering heme toxicity.
Keywords: Staphylococcus aureus; heme synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

