Myosin expression and contractile function are altered by replating stem cell-derived cardiomyocytes
- PMID: 37656049
- PMCID: PMC10473967
- DOI: 10.1085/jgp.202313377
Myosin expression and contractile function are altered by replating stem cell-derived cardiomyocytes
Abstract
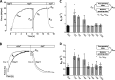
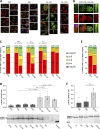
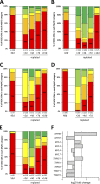
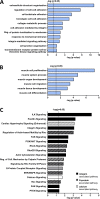
Myosin heavy chain (MyHC) is the main determinant of contractile function. Human ventricular cardiomyocytes (CMs) predominantly express the β-isoform. We previously demonstrated that ∼80% of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) express exclusively β-MyHC after long-term culture on laminin-coated glass coverslips. Here, we investigated the impact of enzymatically detaching hESC-CMs after long-term culture and subsequently replating them for characterization of cellular function. We observed that force-related kinetic parameters, as measured in a micromechanical setup, resembled α- rather than β-MyHC-expressing myofibrils, as well as changes in calcium transients. Single-cell immunofluorescence analysis revealed that replating hESC-CMs led to rapid upregulation of α-MyHC, as indicated by increases in exclusively α-MyHC- and in mixed α/β-MyHC-expressing hESC-CMs. A comparable increase in heterogeneity of MyHC isoform expression was also found among individual human induced pluripotent stem cell (hiPSC)-derived CMs after replating. Changes in MyHC isoform expression and cardiomyocyte function induced by replating were reversible in the course of the second week after replating. Gene enrichment analysis based on RNA-sequencing data revealed changes in the expression profile of mechanosensation/-transduction-related genes and pathways, especially integrin-associated signaling. Accordingly, the integrin downstream mediator focal adhesion kinase (FAK) promoted β-MyHC expression on a stiff matrix, further validating gene enrichment analysis. To conclude, detachment and replating induced substantial changes in gene expression, MyHC isoform composition, and function of long-term cultivated human stem cell-derived CMs, thus inducing alterations in mechanosensation/-transduction, that need to be considered, particularly for downstream in vitro assays.
© 2023 Osten et al.
Conflict of interest statement
Disclosures: T. Thum reported other from Cardior Pharmaceuticals outside the submitted work. No other disclosures were reported.
Figures


Comment in
-
Rethinking replating.J Gen Physiol. 2023 Nov 6;155(11):e202313491. doi: 10.1085/jgp.202313491. Epub 2023 Oct 17. J Gen Physiol. 2023. PMID: 37847309 Free PMC article.
References
-
- Clemente, C.F., Xavier-Neto J., Dalla Costa A.P., Consonni S.R., Antunes J.E., Rocco S.A., Pereira M.B., Judice C.C., Strauss B., Joazeiro P.P., et al. 2012. Focal adhesion kinase governs cardiac concentric hypertrophic growth by activating the AKT and mTOR pathways. J. Mol. Cell. Cardiol. 52:493–501. 10.1016/j.yjmcc.2011.10.015 - DOI - PubMed
-
- Denning, C., Borgdorff V., Crutchley J., Firth K.S., George V., Kalra S., Kondrashov A., Hoang M.D., Mosqueira D., Patel A., et al. 2016. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta. 1863:1728–1748. 10.1016/j.bbamcr.2015.10.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

