Treatment consistent with idiopathic multicentric Castleman disease guidelines is associated with improved outcomes
- PMID: 37656441
- PMCID: PMC10637880
- DOI: 10.1182/bloodadvances.2023010745
Treatment consistent with idiopathic multicentric Castleman disease guidelines is associated with improved outcomes
Abstract
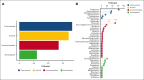
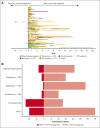
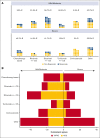
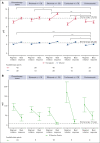
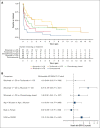
Idiopathic multicentric Castleman disease (iMCD) is a rare hematologic disorder with an unknown etiology. Clinical presentation is heterogeneous, ranging from mild constitutional symptoms with lymphadenopathy to life-threatening multiorgan dysfunction. International, consensus treatment guidelines developed in 2018 relied upon a limited number of clinical trials and small case series; however, to our knowledge, real-world performance of these recommendations has not been subsequently studied. Siltuximab, a monoclonal antibody against interleukin 6 (IL6), is approved for the treatment of iMCD and recommended first-line, and tocilizumab, a monoclonal antibody directed against the IL6 receptor, is recommended when siltuximab is unavailable. Chemotherapy, rituximab, and immunomodulators are recommended as second- and third-line treatments based on limited evidence. Corticosteroid monotherapy is used by clinicians, although not recommended. Here, we draw upon the ACCELERATE Natural History Registry to inventory regimens and evaluate regimen response for 102 expert-confirmed iMCD cases. Siltuximab with/without (w/wo) corticosteroids was associated with a 52% response, whereas corticosteroid monotherapy was associated with a 3% response. Anti-IL6-directed therapy with siltuximab or tocilizumab demonstrated better response and more durability than was observed with rituximab w/wo corticosteroids. Cytotoxic chemotherapy was associated with a 52% response and was predominantly administered in patients characterized by thrombocytopenia, anasarca, fever, renal failure/reticulin fibrosis, and organomegaly. Our results provide evidence in support of current recommendations to administer anti-IL6 as first-line treatment, to administer cytotoxic chemotherapy in patients with severe refractory disease, and to limit corticosteroid monotherapy. Evidence remains limited for effective agents for patients who are refractory to anti-IL6-directed therapy. This trial was registered at www.clinicaltrials.gov as #NCT02817997.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: D.C.F. has received research funding for the ACCELERATE Registry and consulting fees from EUSA Pharma; has received research study drug, with no associated research funding, for the clinical trial of sirolimus from Pfizer (NCT03933904); and has 2 provisional patents pending related to the diagnosis and treatment of iMCD, including 1 related to CXCL13 as a biomarker in iMCD. G.S. has received speaker’s bureau fees from Takeda, Janssen Pharmaceuticals, Foundation Medicine, and EUSA Pharma. J.D.B. has received consulting fees from EUSA Pharma. F.v.R. has received consulting fees from EUSA Pharma, GlaxoSmithKline, Karyopharm, and Takeda; and has received research funding from Janssen Pharmaceuticals and Bristol Myers Squibb. C.C has received consulting fees from EUSA Pharma. The remaining authors report no competing financial interests.
Figures

References
-
- Beck JT, Hsu SM, Wijdenes J, et al. Brief report: alleviation of systemic manifestations of Castleman’s disease by monoclonal anti-interleukin-6 antibody. N Engl J Med. 1994;330(9):602–605. - PubMed
-
- Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74(4):1360–1367. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

