Genomic Sequencing as a First-Tier Screening Test and Outcomes of Newborn Screening
- PMID: 37656460
- PMCID: PMC10474521
- DOI: 10.1001/jamanetworkopen.2023.31162
Genomic Sequencing as a First-Tier Screening Test and Outcomes of Newborn Screening
Abstract
Importance: Newborn screening via biochemical tests is in use worldwide. The availability of genetic sequencing has allowed rapid screening for a substantial number of monogenic disorders. However, the outcomes of this strategy have not been evaluated in a general newborn population.
Objective: To evaluate the outcomes of applying gene panel sequencing as a first-tier newborn screening test.
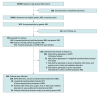
Design, setting, and participants: This cohort study included newborns who were prospectively recruited from 8 screening centers in China between February 21 and December 31, 2021. Neonates with positive results were followed up before July 5, 2022.
Exposures: All participants were concurrently screened using dried blood spots. The screen consisted of biochemical screening tests and a targeted gene panel sequencing test for 128 conditions. The biochemical and genomic tests could both detect 43 of the conditions, whereas the other 85 conditions were screened solely by the gene panel.
Main outcomes and measures: The primary outcomes were the number of patients detected by gene panel sequencing but undetected by the biochemical test.
Results: This study prospectively recruited 29 601 newborns (15 357 [51.2%] male). The mean (SD) gestational age was 39.0 (1.5) weeks, and the mean (SD) birth weight was 3273 (457) g. The gene panel sequencing screened 813 infants (2.7%; 95% CI, 2.6%-2.9%) as positive. By the date of follow-up, 402 infants (1.4%; 95% CI, 1.2%-1.5%) had been diagnosed, indicating the positive predictive value was 50.4% (95% CI, 50.0%-53.9%). The gene panel sequencing identified 59 patients undetected by biochemical tests, including 20 patients affected by biochemically and genetically screened disorders and 39 patients affected by solely genetically screened disorders, which translates into 1 out of every 500 newborns (95% CI, 1/385-1/625) benefiting from the implementation of gene panels as a first-tier screening test.
Conclusions and relevance: In this cohort study, the use of gene panel sequencing in a general newborn population as a first-tier screening test improved the detection capability of traditional screening, providing an evidence-based suggestion that it could be considered as a crucial method for first-tier screening.
Conflict of interest statement
Figures
Comment in
-
Genome Sequencing for Newborn Screening-An Effective Approach for Tackling Rare Diseases.JAMA Netw Open. 2023 Sep 5;6(9):e2331141. doi: 10.1001/jamanetworkopen.2023.31141. JAMA Netw Open. 2023. PMID: 37656463 No abstract available.
Similar articles
-
Newborn Screening for 6 Lysosomal Storage Disorders in China.JAMA Netw Open. 2024 May 1;7(5):e2410754. doi: 10.1001/jamanetworkopen.2024.10754. JAMA Netw Open. 2024. PMID: 38739391 Free PMC article.
-
Diagnosis of Classic Homocystinuria in Two Boys Presenting with Acute Cerebral Venous Thrombosis and Neurologic Dysfunction after Normal Newborn Screening.Int J Neonatal Screen. 2021 Jul 23;7(3):48. doi: 10.3390/ijns7030048. Int J Neonatal Screen. 2021. PMID: 34449521 Free PMC article.
-
Feasibility of Screening for Chromosome 15 Imprinting Disorders in 16 579 Newborns by Using a Novel Genomic Workflow.JAMA Netw Open. 2022 Jan 4;5(1):e2141911. doi: 10.1001/jamanetworkopen.2021.41911. JAMA Netw Open. 2022. PMID: 34982160 Free PMC article.
-
Universal newborn hearing screening: summary of evidence.JAMA. 2001 Oct 24-31;286(16):2000-10. doi: 10.1001/jama.286.16.2000. JAMA. 2001. PMID: 11667937 Review.
-
Identifying Non-Duchenne Muscular Dystrophy-Positive and False Negative Results in Prior Duchenne Muscular Dystrophy Newborn Screening Programs: A Review.JAMA Neurol. 2016 Jan;73(1):111-6. doi: 10.1001/jamaneurol.2015.3537. JAMA Neurol. 2016. PMID: 26594870 Review.
Cited by
-
Harnessing Next-Generation Sequencing as a Timely and Accurate Second-Tier Screening Test for Newborn Screening of Inborn Errors of Metabolism.Int J Neonatal Screen. 2024 Mar 5;10(1):19. doi: 10.3390/ijns10010019. Int J Neonatal Screen. 2024. PMID: 38535123 Free PMC article.
-
Progress of newborn screening in China.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023 Dec 19;52(6):673-682. doi: 10.3724/zdxbyxb-2023-0467. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 38115737 Free PMC article. Chinese, English.
-
Data-driven consideration of genetic disorders for global genomic newborn screening programs.Genet Med. 2025 Jul;27(7):101443. doi: 10.1016/j.gim.2025.101443. Epub 2025 May 9. Genet Med. 2025. PMID: 40357684
-
Expanding implementation of pediatric whole-genome sequencing: Insights from SeqFirst providers to inform equitable access to a precise genetic diagnosis.HGG Adv. 2025 Jun 3;6(4):100464. doi: 10.1016/j.xhgg.2025.100464. Online ahead of print. HGG Adv. 2025. PMID: 40468597 Free PMC article.
-
Newborn Screening by DNA-First: Systematic Evaluation of the Eligibility of Inherited Metabolic Disorders Based on Treatability.Int J Neonatal Screen. 2024 Dec 28;11(1):1. doi: 10.3390/ijns11010001. Int J Neonatal Screen. 2024. PMID: 39846587 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical