The atypical 'hippocampal' glutamate receptor coupled to phospholipase D that controls stretch-sensitivity in primary mechanosensory nerve endings is homomeric purely metabotropic GluK2
- PMID: 37656490
- PMCID: PMC10988755
- DOI: 10.1113/EP090761
The atypical 'hippocampal' glutamate receptor coupled to phospholipase D that controls stretch-sensitivity in primary mechanosensory nerve endings is homomeric purely metabotropic GluK2
Abstract
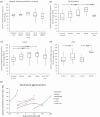
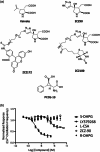
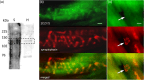
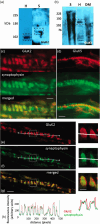
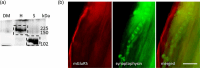
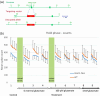
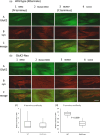
A metabotropic glutamate receptor coupled to phospholipase D (PLD-mGluR) was discovered in the hippocampus over three decades ago. Its pharmacology and direct linkage to PLD activation are well established and indicate it is a highly atypical glutamate receptor. A receptor with the same pharmacology is present in spindle primary sensory terminals where its blockade can totally abolish, and its activation can double, the normal stretch-evoked firing. We report here the first identification of this PLD-mGluR protein, by capitalizing on its expression in primary mechanosensory terminals, developing an enriched source, pharmacological profiling to identify an optimal ligand, and then functionalizing it as a molecular tool. Evidence from immunofluorescence, western and far-western blotting indicates PLD-mGluR is homomeric GluK2, since GluK2 is the only glutamate receptor protein/receptor subunit present in spindle mechanosensory terminals. Its expression was also found in the lanceolate palisade ending of hair follicle, also known to contain the PLD-mGluR. Finally, in a mouse model with ionotropic function ablated in the GluK2 subunit, spindle glutamatergic responses were still present, confirming it acts purely metabotropically. We conclude the PLD-mGluR is a homomeric GluK2 kainate receptor signalling purely metabotropically and it is common to other, perhaps all, primary mechanosensory endings.
Keywords: GluK2; PLD-mGluR; glutamate receptor; kainate receptor; mechanosensation; muscle spindle.
© 2023 The Authors. Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
H.E.S., B.A.H., L.M.B. and D.B. were employees of Eli Lilly at the time the work was done, and Eli Lilly joint‐funded the scholarship for S.W.’s contribution to this study.
Figures



References
-
- Albani‐Torregrossa, S. , Attucci, S. , Marinozzi, M. , Pellicciari, R. , Moroni, F. , & Pellegrini‐Giampietro, D. E. (1999). Antagonist pharmacology of metabotropic glutamate receptors coupled to phospholipase D activation in adult rat hippocampus: Focus on (2R,1'S,2'R,3'S)‐2‐(2'‐carboxy‐3'‐phenylcyclopropyl)glycine versus 3, 5‐dihydroxyphenylglycine. Molecular Pharmacology, 55, 699–707. - PubMed
-
- Banks, R. W. , Bewick, G. S. , Reid, B. , & Richardson, C. (2002). Evidence for activity‐dependent modulation of sensory‐terminal excitability in spindles by glutamate release from synaptic‐like vesicles. Advances in Experimental Medicine and Biology, 508, 13–18. - PubMed
-
- Banks, R. W. , Cahusac, P. M. B. , Graca, A. , Kain, N. , Shenton, F. , Singh, P. , Njå, A. , Simon, A. , Watson, S. , Slater, C. R. , & Bewick, G. S. (2013). Glutamatergic modulation of synaptic‐like vesicle recycling in mechanosensory lanceolate nerve terminals of mammalian hair follicles. The Journal of Physiology, 591(10), 2523–2540. - PMC - PubMed
-
- Béhé, P. , Stern, P. , Wyllie, D. J. , Nassar, M. , Schoepfer, R. , & Colquhoun, D. (1995). Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proceedings of the Royal Society B: Biological Sciences, 262, 205–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
