Comparison of collection methods for Phlebotomus argentipes sand flies to use in a molecular xenomonitoring system for the surveillance of visceral leishmaniasis
- PMID: 37656745
- PMCID: PMC10501600
- DOI: 10.1371/journal.pntd.0011200
Comparison of collection methods for Phlebotomus argentipes sand flies to use in a molecular xenomonitoring system for the surveillance of visceral leishmaniasis
Abstract
Background: The kala-azar elimination programme has resulted in a significant reduction in visceral leishmaniasis (VL) cases across the Indian Subcontinent. To detect any resurgence of transmission, a sensitive cost-effective surveillance system is required. Molecular xenomonitoring (MX), detection of pathogen DNA/RNA in vectors, provides a proxy of human infection in the lymphatic filariasis elimination programme. To determine whether MX can be used for VL surveillance in a low transmission setting, large numbers of the sand fly vector Phlebotomus argentipes are required. This study will determine the best method for capturing P. argentipes females for MX.
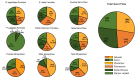
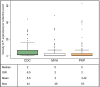
Methodology/principal findings: The field study was performed in two programmatic and two non-programmatic villages in Bihar, India. A total of 48 households (12/village) were recruited. Centers for Disease Control and Prevention light traps (CDC-LTs) were compared with Improved Prokopack (PKP) and mechanical vacuum aspirators (MVA) using standardised methods. Four 12x12 Latin squares, 576 collections, were attempted (12/house, 144/village,192/method). Molecular analyses of collections were conducted to confirm identification of P. argentipes and to detect human and Leishmania DNA. Operational factors, such as time burden, acceptance to householders and RNA preservation, were also considered. A total of 562 collections (97.7%) were completed with 6,809 sand flies captured. Females comprised 49.0% of captures, of which 1,934 (57.9%) were identified as P. argentipes. CDC-LTs collected 4.04 times more P. argentipes females than MVA and 3.62 times more than PKP (p<0.0001 for each). Of 21,735 mosquitoes in the same collections, no significant differences between collection methods were observed. CDC-LTs took less time to install and collect than to perform aspirations and their greater yield compensated for increased sorting time. No significant differences in Leishmania RNA detection and quantitation between methods were observed in experimentally infected sand flies maintained in conditions simulating field conditions. CDC-LTs were favoured by householders.
Conclusions/significance: CDC-LTs are the most useful collection tool of those tested for MX surveillance since they collected higher numbers of P. argentipes females without compromising mosquito captures or the preservation of RNA. However, capture rates are still low.
Copyright: © 2023 McIntyre-Nolan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- World Health Organization. Regional Office for South-East Asia. Regional strategic framework for elimination of kala azar from the South-East Asia Region (2005–2015). New Delhi: WHO Regional Office for South-East Asia; 2005. Available from: https://apps.who.int/iris/handle/10665/205825
-
- National Vector Borne Disease Control Programme. Accelerated plan for kala-azar elimination. Delhi, India: NVBDCP; 2017. Available from: https://nvbdcp.gov.in/WriteReadData/l892s/Accelerated-Plan-Kala-azar1-Fe...
-
- World Health Organization. Global leishmaniasis surveillance, 2017–2018, and first report on 5 additional indicators. Weekly Epidemiological Record. 2020;95:265–280. Available from: https://www.who.int/publications/i/item/who-wer9525
-
- World Health Organization. Report of Meeting of the Regional Technical Advisory Group (RTAG) on Visceral Leishmaniasis and the National Visceral Leishmaniasis Programme Managers of endemic Member States Regional Office for South-East Asia; 2020. Available from: https://apps.who.int/iris/handle/10665/340612
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

