A Status Report on "Gold Standard" Machine-Learned Potentials for Water
- PMID: 37656898
- PMCID: PMC10510435
- DOI: 10.1021/acs.jpclett.3c01791
A Status Report on "Gold Standard" Machine-Learned Potentials for Water
Abstract
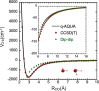
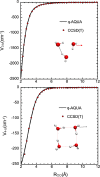
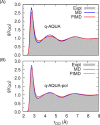
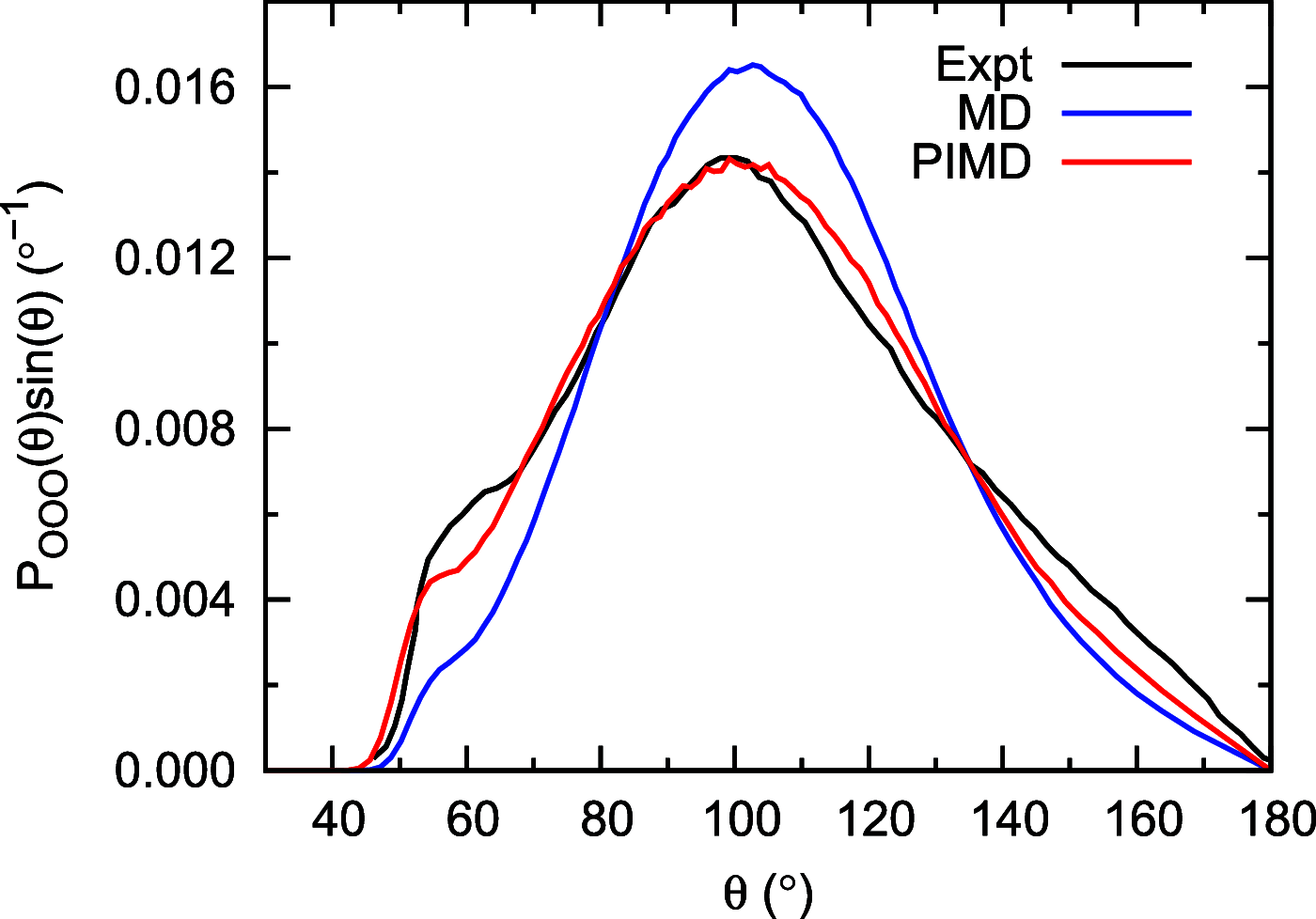
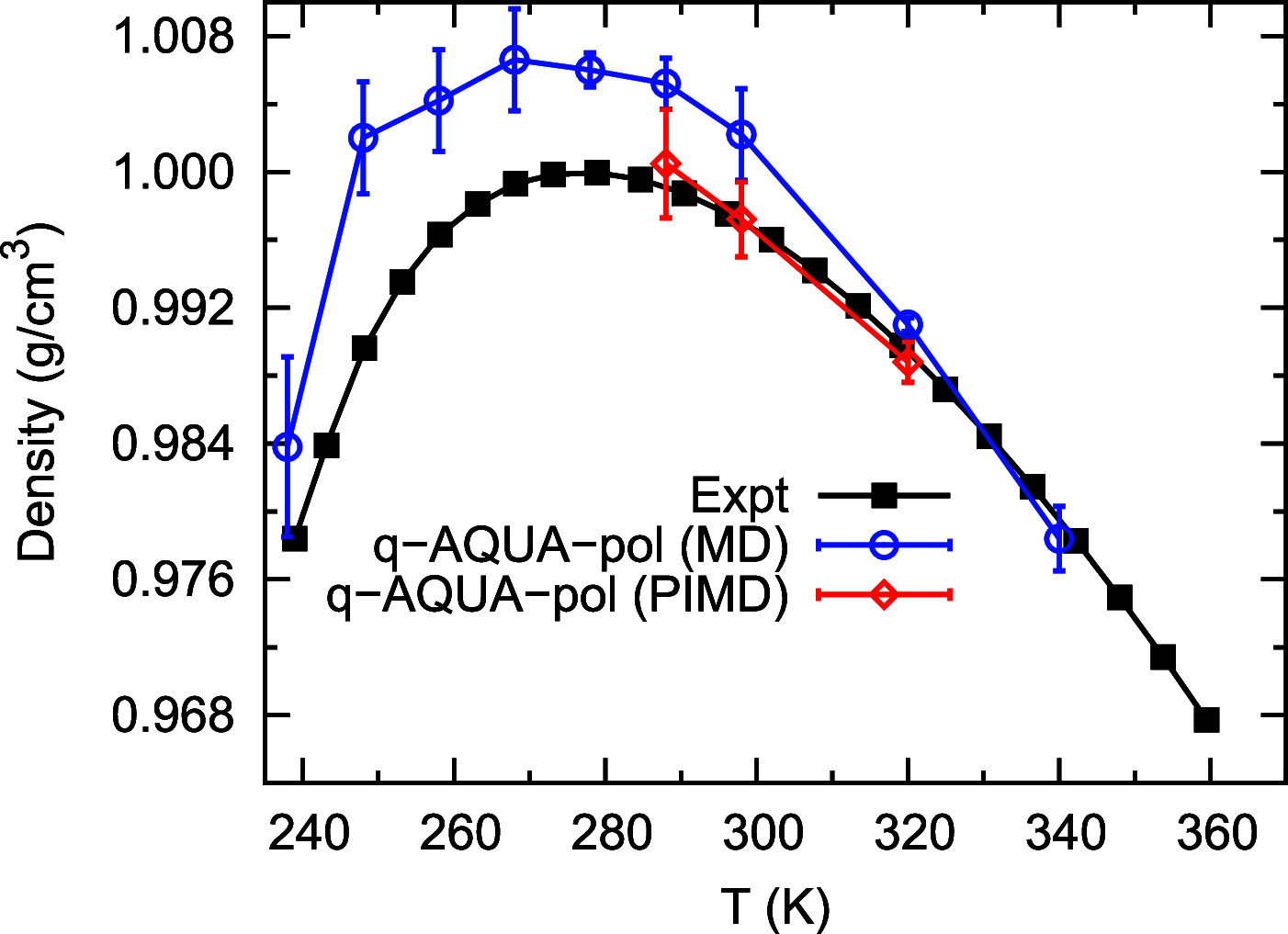
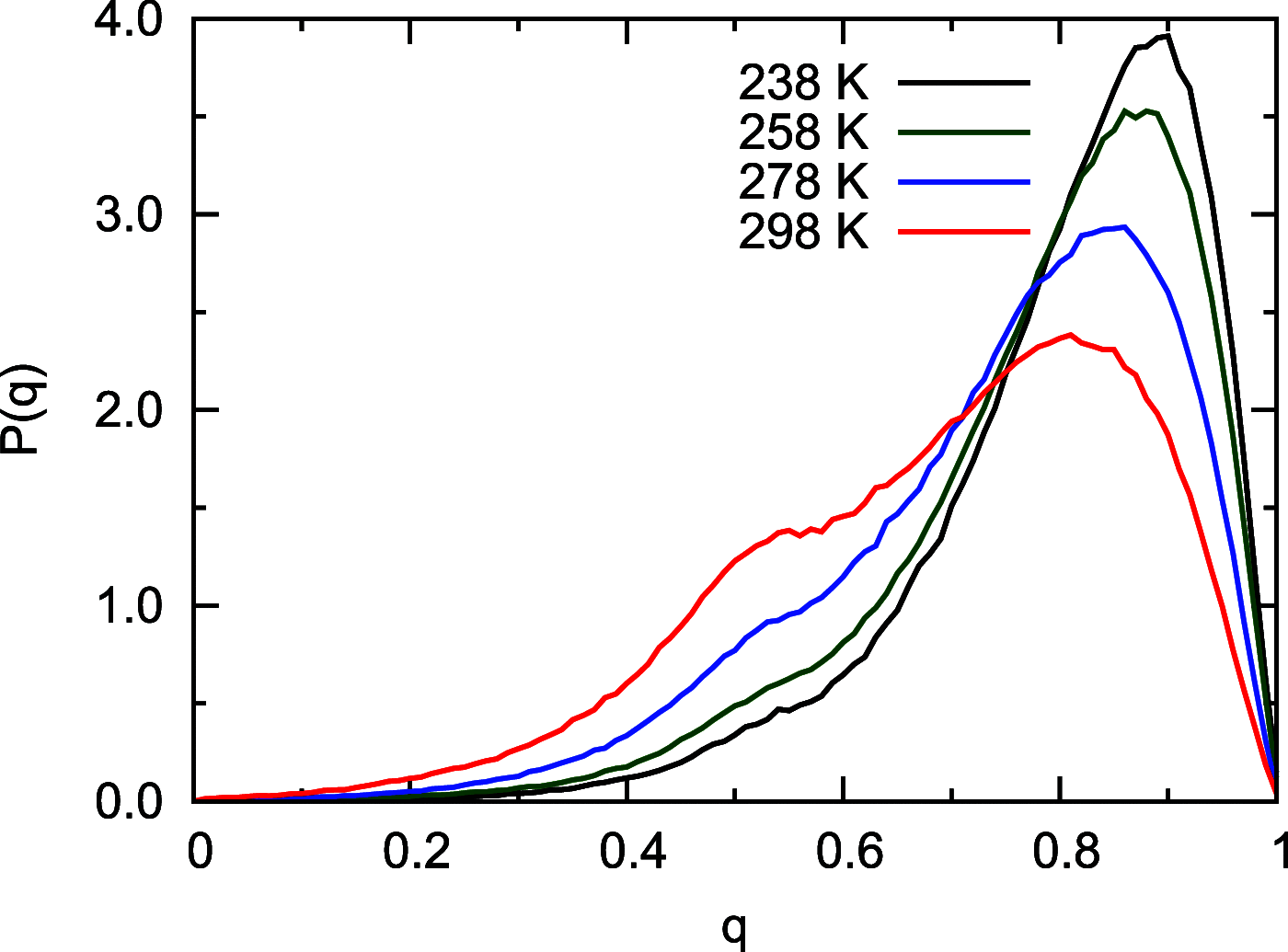
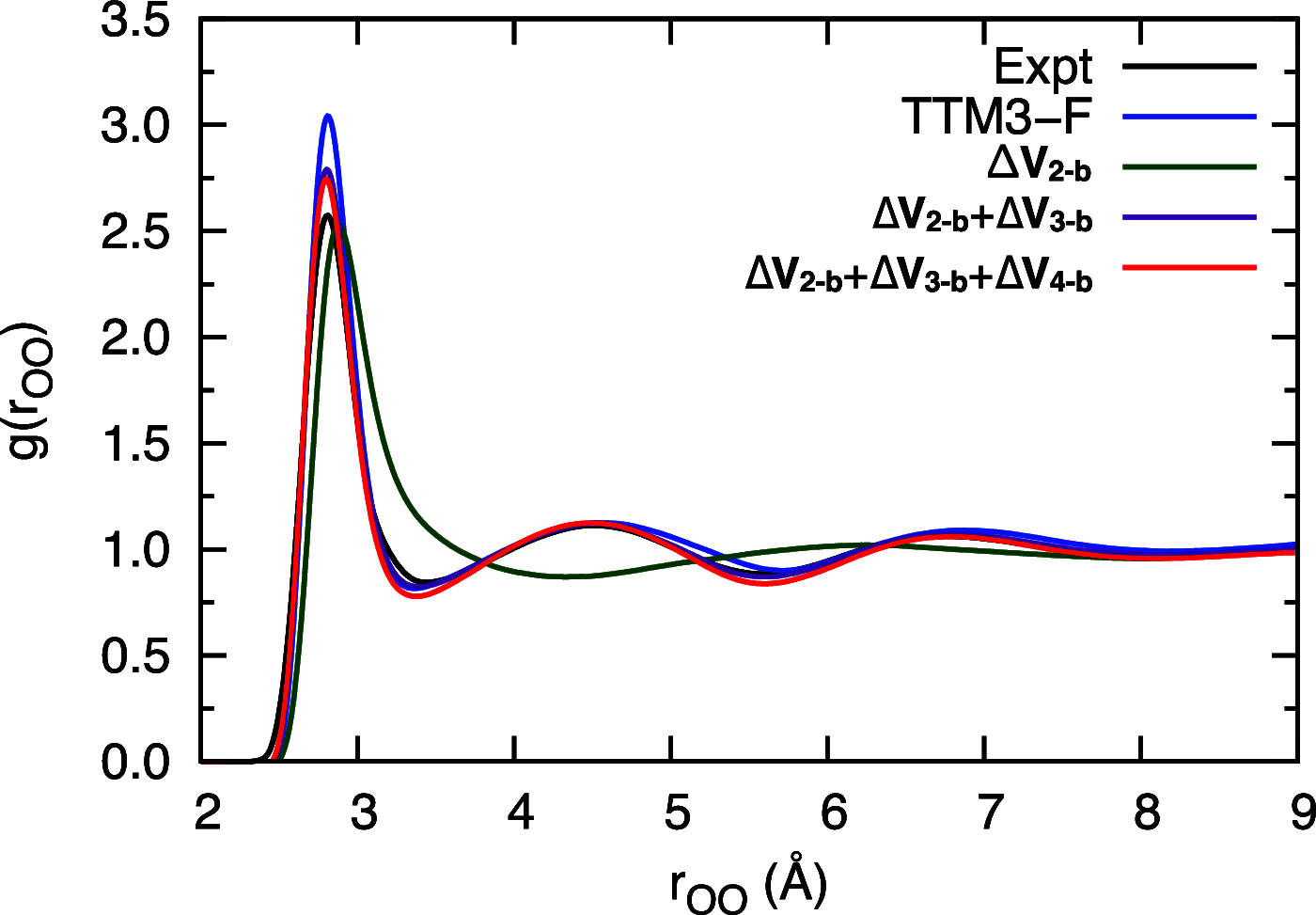
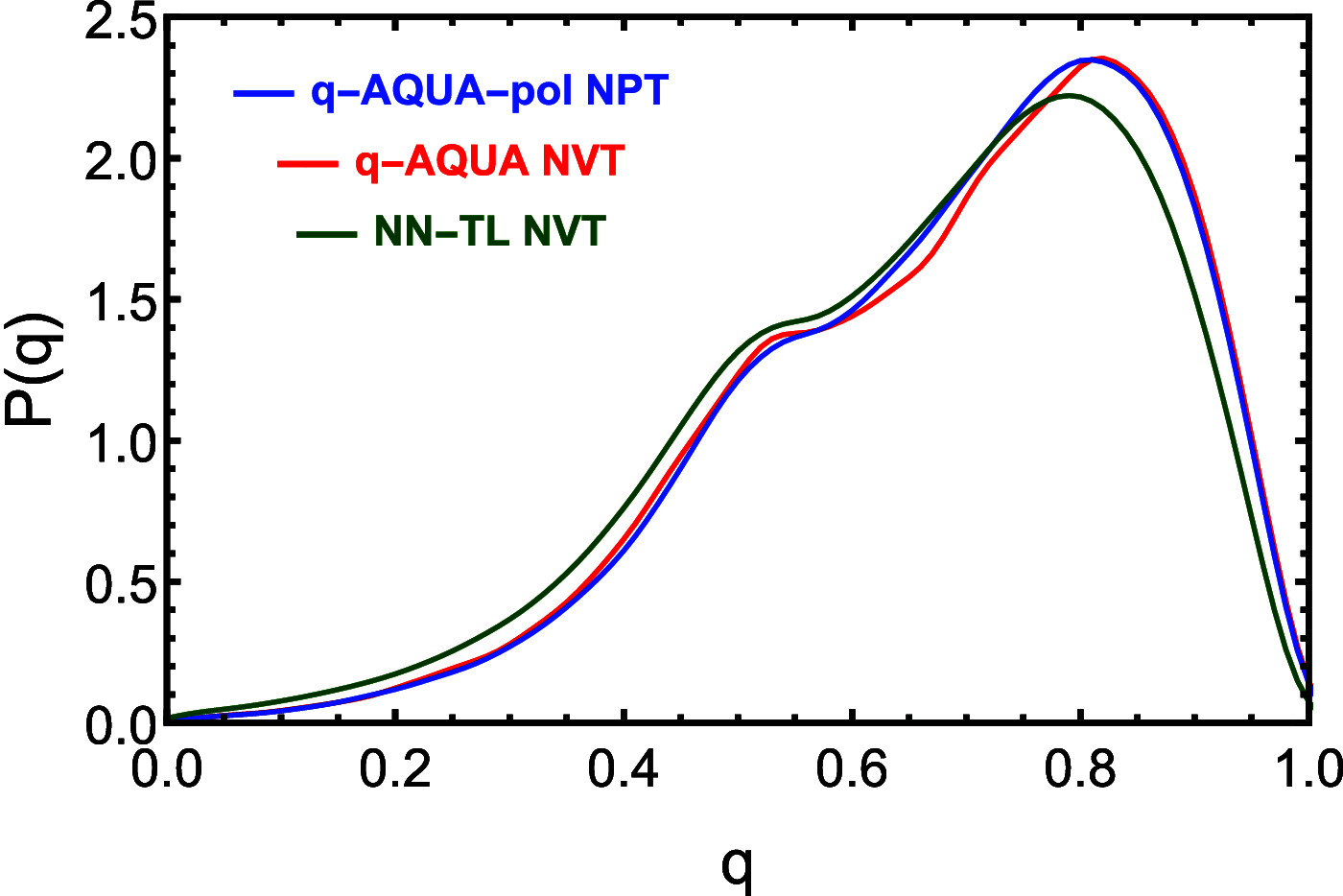
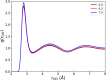
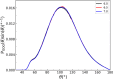
Owing to the central importance of water to life as well as its unusual properties, potentials for water have been the subject of extensive research over the past 50 years. Recently, five potentials based on different machine learning approaches have been reported that are at or near the "gold standard" CCSD(T) level of theory. The development of such high-level potentials enables efficient and accurate simulations of water systems using classical and quantum dynamical approaches. This Perspective serves as a status report of these potentials, focusing on their methodology and applications to water systems across different phases. Their performances on the energies of gas phase water clusters, as well as condensed phase structural and dynamical properties, are discussed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures