Inhibiting metabotropic glutamate receptor 5 after stroke restores brain function and connectivity
- PMID: 37656990
- PMCID: PMC10766240
- DOI: 10.1093/brain/awad293
Inhibiting metabotropic glutamate receptor 5 after stroke restores brain function and connectivity
Abstract
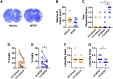
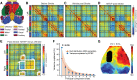
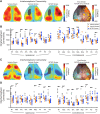
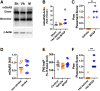
Stroke results in local neural disconnection and brain-wide neuronal network dysfunction leading to neurological deficits. Beyond the hyper-acute phase of ischaemic stroke, there is no clinically-approved pharmacological treatment that alleviates sensorimotor impairments. Functional recovery after stroke involves the formation of new or alternative neuronal circuits including existing neural connections. The type-5 metabotropic glutamate receptor (mGluR5) has been shown to modulate brain plasticity and function and is a therapeutic target in neurological diseases outside of stroke. We investigated whether mGluR5 influences functional recovery and network reorganization rodent models of focal ischaemia. Using multiple behavioural tests, we observed that treatment with negative allosteric modulators (NAMs) of mGluR5 (MTEP, fenobam and AFQ056) for 12 days, starting 2 or 10 days after stroke, restored lost sensorimotor functions, without diminishing infarct size. Recovery was evident within hours after initiation of treatment and progressed over the subsequent 12 days. Recovery was prevented by activation of mGluR5 with the positive allosteric modulator VU0360172 and accelerated in mGluR5 knock-out mice compared with wild-type mice. After stroke, multisensory stimulation by enriched environments enhanced recovery, a result prevented by VU0360172, implying a role of mGluR5 in enriched environment-mediated recovery. Additionally, MTEP treatment in conjunction with enriched environment housing provided an additive recovery enhancement compared to either MTEP or enriched environment alone. Using optical intrinsic signal imaging, we observed brain-wide disruptions in resting-state functional connectivity after stroke that were prevented by mGluR5 inhibition in distinct areas of contralesional sensorimotor and bilateral visual cortices. The levels of mGluR5 protein in mice and in tissue samples of stroke patients were unchanged after stroke. We conclude that neuronal circuitry subserving sensorimotor function after stroke is depressed by a mGluR5-dependent maladaptive plasticity mechanism that can be restored by mGluR5 inhibition. Post-acute stroke treatment with mGluR5 NAMs combined with rehabilitative training may represent a novel post-acute stroke therapy.
Keywords: long term depression; pharmacological therapy; plasticity; resting-state functional connectivity; stroke recovery.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
T.W., R.O., K.R. and C.S. are co-inventors on a patent related to the content of this article. The other authors report no competing interests.
Figures




References
-
- Carey LM, Matyas TA. Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. J Rehabil Med. 2011;43:257–263. - PubMed
-
- Winstein CJ, Stein J, Arena R, et al. . Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:e98–e169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

