CFTR potentiator ivacaftor protects against noise-induced hair cell loss by increasing Nrf2 and reducing oxidative stress
- PMID: 37657258
- PMCID: PMC10528730
- DOI: 10.1016/j.biopha.2023.115399
CFTR potentiator ivacaftor protects against noise-induced hair cell loss by increasing Nrf2 and reducing oxidative stress
Abstract
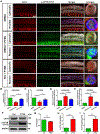
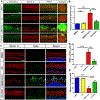
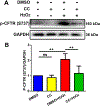
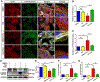
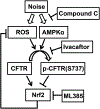
Over-production of reactive oxygen species (ROS) in the inner ear can be triggered by a variety of pathological events identified in animal models after traumatic noise exposure. Our previous research found that inhibition of the AMP-activated protein kinase alpha subunit (AMPKα) protects against noise-induced cochlear hair cell loss and hearing loss by reducing ROS accumulation. However, the molecular pathway through which AMPKα exerts its antioxidative effect is still unclear. In this study, we have investigated a potential target of AMPKα and ROS, cystic fibrosis transmembrane conductance regulator (CFTR), and the protective effect against noise-induced hair cell loss of an FDA-approved CFTR potentiator, ivacaftor, in FVB/NJ mice, mouse explant cultures, and HEI-OC1 cells. We found that noise exposure increases phosphorylation of CFTR at serine 737 (p-CFTR, S737), which reduces wildtype CFTR function, resulting in oxidative stress in cochlear sensory hair cells. Pretreatment with a single dose of ivacaftor maintains CFTR function by preventing noise-increased p-CFTR (S737). Furthermore, ivacaftor treatment increases nuclear factor E2-related factor 2 (Nrf2) expression, diminishes ROS formation, and attenuates noise-induced hair cell loss and hearing loss. Additionally, inhibition of noise-induced AMPKα activation by compound C also diminishes p-CFTR (S737) expression. In line with these in-vivo results, administration of hydrogen peroxide to cochlear explants or HEI-OC1 cells increases p-CFTR (S737) expression and induces sensory hair cell or HEI-OC1 cell damage, while application of ivacaftor halts these effects. Although ivacaftor increases Nrf2 expression and reduces ROS accumulation, cotreatment with ML385, an Nrf2 inhibitor, abolishes the protective effects of ivacaftor against hydrogen-peroxide-induced HEI-OC1 cell death. Our results indicate that noise-induced sensory hair cell damage is associated with p-CFTR. Ivacaftor has potential for treatment of noise-induced hearing loss by maintaining CFTR function and increasing Nrf2 expression for support of redox homeostasis in sensory hair cells.
Keywords: Activation of AMPKα; Cystic fibrosis transmembrane conductance regulator ivacaftor; Noise-induced hearing loss; Nuclear factor E2-related factor 2; Reactive oxygen species.
Copyright © 2023 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no competing interests.
Figures






Similar articles
-
Ivacaftor attenuates gentamicin-induced ototoxicity through the CFTR-Nrf2-HO1/NQO1 pathway.Redox Rep. 2024 Dec;29(1):2332038. doi: 10.1080/13510002.2024.2332038. Epub 2024 Apr 2. Redox Rep. 2024. PMID: 38563333 Free PMC article.
-
Deferoxamine protects cochlear hair cells and hair cell-like HEI-OC1 cells against tert-butyl hydroperoxide-induced ototoxicity.Biochim Biophys Acta Mol Basis Dis. 2024 Mar;1870(3):167024. doi: 10.1016/j.bbadis.2024.167024. Epub 2024 Jan 17. Biochim Biophys Acta Mol Basis Dis. 2024. PMID: 38242180
-
Noise-Induced Loss of Hair Cells and Cochlear Synaptopathy Are Mediated by the Activation of AMPK.J Neurosci. 2016 Jul 13;36(28):7497-510. doi: 10.1523/JNEUROSCI.0782-16.2016. J Neurosci. 2016. PMID: 27413159 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment.Ann Pharmacother. 2012 Jul-Aug;46(7-8):1065-75. doi: 10.1345/aph.1R076. Epub 2012 Jun 26. Ann Pharmacother. 2012. PMID: 22739718 Review.
-
Ivacaftor for the treatment of cystic fibrosis in children under six years of age.Expert Rev Respir Med. 2020 Jun;14(6):547-557. doi: 10.1080/17476348.2020.1741352. Epub 2020 Mar 17. Expert Rev Respir Med. 2020. PMID: 32154747 Review.
Cited by
-
Ivacaftor attenuates gentamicin-induced ototoxicity through the CFTR-Nrf2-HO1/NQO1 pathway.Redox Rep. 2024 Dec;29(1):2332038. doi: 10.1080/13510002.2024.2332038. Epub 2024 Apr 2. Redox Rep. 2024. PMID: 38563333 Free PMC article.
-
Exploring AAV-Mediated Gene Therapy for Inner Ear Diseases: from Preclinical Success to Clinical Potential.Adv Sci (Weinh). 2025 Sep;12(33):e08397. doi: 10.1002/advs.202408397. Epub 2025 Jun 20. Adv Sci (Weinh). 2025. PMID: 40538303 Free PMC article. Review.
-
Hesperidin activates Nrf2 to protect cochlear hair cells from cisplatin-induced damage.Redox Rep. 2024 Dec;29(1):2341470. doi: 10.1080/13510002.2024.2341470. Epub 2024 Apr 17. Redox Rep. 2024. PMID: 38629504 Free PMC article.
-
AAVR Expression is Essential for AAV Vector Transduction in Sensory Hair Cells.Adv Sci (Weinh). 2025 Aug;12(29):e2408873. doi: 10.1002/advs.202408873. Epub 2025 Jan 7. Adv Sci (Weinh). 2025. PMID: 39776318 Free PMC article.
References
-
- Varela-Nieto I, Murillo-Cuesta S, Calvino M, Cediel R, Lassaletta L, Drug development for noise-induced hearing loss, Expert Opin. Drug Discov. 15 (12) (2020) 1457–1471. - PubMed
-
- Hudspeth AJ, Integrating the active process of hair cells with cochlear function, Nat. Rev. Neurosci 15 (9) (2014) 600–614. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

