Targeting nucleic acid sensors in tumor cells to reprogram biogenesis and RNA cargo of extracellular vesicles for T cell-mediated cancer immunotherapy
- PMID: 37657445
- PMCID: PMC10518594
- DOI: 10.1016/j.xcrm.2023.101171
Targeting nucleic acid sensors in tumor cells to reprogram biogenesis and RNA cargo of extracellular vesicles for T cell-mediated cancer immunotherapy
Abstract
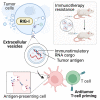
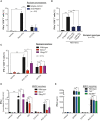
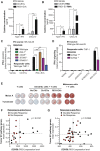
Tumor-derived extracellular vesicles (EVs) have been associated with immune evasion and tumor progression. We show that the RNA-sensing receptor RIG-I within tumor cells governs biogenesis and immunomodulatory function of EVs. Cancer-intrinsic RIG-I activation releases EVs, which mediate dendritic cell maturation and T cell antitumor immunity, synergizing with immune checkpoint blockade. Intact RIG-I, autocrine interferon signaling, and the GTPase Rab27a in tumor cells are required for biogenesis of immunostimulatory EVs. Active intrinsic RIG-I signaling governs composition of the tumor EV RNA cargo including small non-coding stimulatory RNAs. High transcriptional activity of EV pathway genes and RIG-I in melanoma samples associate with prolonged patient survival and beneficial response to immunotherapy. EVs generated from human melanoma after RIG-I stimulation induce potent antigen-specific T cell responses. We thus define a molecular pathway that can be targeted in tumors to favorably alter EV immunomodulatory function. We propose "reprogramming" of tumor EVs as a personalized strategy for T cell-mediated cancer immunotherapy.
Keywords: RIG-I; RNA; STING; cancer immunotherapy; cancer resistance; extracellular vesicles; innate immunity; nucleic acid receptors; personalized therapy.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.H. has been a consultant for Bristol Myers-Squibb (BMS), Novartis, Merck, Abbvie, and Roche, has received research funding from BMS and Novartis, and is an employee of and holds equity interest in Roche/Genentech. A.G. is a consultant for and has equity interest in Evox Therapeutics Ltd. and is inventor on several patent applications related to extracellular vesicles. B.G. is a scientific advisory board member of Innovex Therapeutics SL, PL BioScience, and Mursla Ltd, consultant for FUJIFILM Wako Chemicals, and a founding director of Exosla Ltd. G.H. is inventor on a patent covering synthetic RIG-I ligand, and co-founder of Rigontec GmbH. H.P. is a consultant for Gilead, Abbvie, Pfizer, Novartis, Servier, and BMS, and has received research funding from BMS.
Figures




References
-
- Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., Li X.D., Mauceri H., Beckett M., Darga T., et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. - DOI - PMC - PubMed
-
- Fischer J.C., Bscheider M., Eisenkolb G., Lin C.C., Wintges A., Otten V., Lindemans C.A., Heidegger S., Rudelius M., Monette S., et al. RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury. Sci. Transl. Med. 2017;9:eaag2513. doi: 10.1126/scitranslmed.aag2513. - DOI - PMC - PubMed
-
- Woo S.R., Fuertes M.B., Corrales L., Spranger S., Furdyna M.J., Leung M.Y.K., Duggan R., Wang Y., Barber G.N., Fitzgerald K.A., et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

