Phenotypes of Disseminated Intravascular Coagulation
- PMID: 37657485
- PMCID: PMC10890912
- DOI: 10.1055/a-2165-1142
Phenotypes of Disseminated Intravascular Coagulation
Abstract
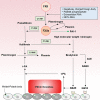
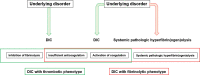
Two phenotypes of disseminated intravascular coagulation (DIC) are systematically reviewed. DIC is classified into thrombotic and fibrinolytic phenotypes characterized by thrombosis and hemorrhage, respectively. Major pathology of DIC with thrombotic phenotype is the activation of coagulation, insufficient anticoagulation with endothelial injury, and plasminogen activator inhibitor-1-mediated inhibition of fibrinolysis, leading to microvascular fibrin thrombosis and organ dysfunction. DIC with fibrinolytic phenotype is defined as massive thrombin generation commonly observed in any type of DIC, combined with systemic pathologic hyperfibrinogenolysis caused by underlying disorder that results in severe bleeding due to excessive plasmin formation. Three major pathomechanisms of systemic hyperfibrinogenolysis have been considered: (1) acceleration of tissue-type plasminogen activator (t-PA) release from hypoxic endothelial cells and t-PA-rich storage pools, (2) enhancement of the conversion of plasminogen to plasmin due to specific proteins and receptors that are expressed on cancer cells and endothelial cells, and (3) alternative pathways of fibrinolysis. DIC with fibrinolytic phenotype can be diagnosed by DIC diagnosis followed by the recognition of systemic pathologic hyperfibrin(ogen)olysis. Low fibrinogen levels, high fibrinogen and fibrin degradation products (FDPs), and the FDP/D-dimer ratio are important for the diagnosis of systemic pathologic hyperfibrin(ogen)olysis. Currently, evidence-based treatment strategies for DIC with fibrinolytic phenotypes are lacking. Tranexamic acid appears to be one of the few methods to be effective in the treatment of systemic pathologic hyperfibrin(ogen)olysis. International cooperation for the elucidation of pathomechanisms, establishment of diagnostic criteria, and treatment strategies for DIC with fibrinolytic phenotype are urgent issues in the field of thrombosis and hemostasis.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
None declared.
Figures


References
-
- Marder V J, Feinstein D I, Colman R W, Levi M. 5th ed. Philadelphia, PA: Lippincott Wiliams & Wikins; 2006. Consumptive thrombohemorrhagic disorders; pp. 1571–1600.
-
- Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH) . Taylor F B, Jr, Toh C H, Hoots W K, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(05):1327–1330. - PubMed
-
- Gando S, Levi M, Toh C H. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037. - PubMed
-
- Suffredini A F, Harpel P C, Parrillo J E. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. N Engl J Med. 1989;320(18):1165–1172. - PubMed
-
- Levi M, ten Cate H, van der Poll T, van Deventer S J. Pathogenesis of disseminated intravascular coagulation in sepsis. JAMA. 1993;270(08):975–979. - PubMed

