COVID-19-associated pulmonary aspergillosis in mechanically ventilated patients: a prospective, multicentre UK study
- PMID: 37657925
- PMCID: PMC10804023
- DOI: 10.1136/thorax-2023-220002
COVID-19-associated pulmonary aspergillosis in mechanically ventilated patients: a prospective, multicentre UK study
Abstract
Background: Invasive pulmonary aspergillosis is a complication of severe COVID-19, with regional variation in reported incidence and mortality. We describe the incidence, risk factors and mortality associated with COVID-19-associated pulmonary aspergillosis (CAPA) in a prospective, multicentre UK cohort.
Methods: From March 2020 to March 2021, 266 mechanically ventilated adults with COVID-19 were enrolled across 5 UK hospital intensive care units (ICUs). CAPA was defined using European Confederation for Medical Mycology and the International Society for Human and Animal Mycology criteria and fungal diagnostics performed on respiratory and serum samples.
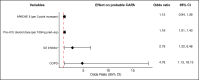
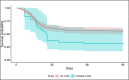
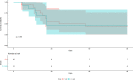
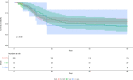
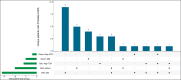
Results: Twenty-nine of 266 patients (10.9%) had probable CAPA, 14 (5.2%) possible CAPA and none proven CAPA. Probable CAPA was diagnosed a median of 9 (IQR 7-16) days after ICU admission. Factors associated with probable CAPA after multivariable logistic regression were cumulative steroid dose given within 28 days prior to ICU admission (adjusted OR (aOR) 1.16; 95% CI 1.01 to 1.43 per 100 mg prednisolone-equivalent), receipt of an interleukin (IL)-6 inhibitor (aOR 2.79; 95% CI 1.22 to 6.48) and chronic obstructive pulmonary disease (COPD) (aOR 4.78; 95% CI 1.13 to 18.13). Mortality in patients with probable CAPA was 55%, vs 46% in those without. After adjustment for immortal time bias, CAPA was associated with an increased risk of 90-day mortality (HR 1.85; 95% CI 1.07 to 3.19); however, this association did not remain statistically significant after further adjustment for confounders (adjusted HR 1.57; 95% CI 0.88 to 2.80). There was no difference in mortality between patients with CAPA prescribed antifungals (9 of 17; 53%) and those who were not (7 of 12; 58%) (p=0.77).
Interpretation: In this first prospective UK study, probable CAPA was associated with corticosteroid use, receipt of IL-6 inhibitors and pre-existing COPD. CAPA did not impact mortality following adjustment for prognostic variables.
Keywords: COVID-19; aspergillus lung disease; critical care; viral infection.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MPW, WH and TB have received speaker fees from Gilead Sciences. JY has received honoraria from Pfizer for contributing to a CAPA working group. TB has received Advisory Board fees from Gilead Sciences and Mundipharma and funding from MSD and Pfizer. DRJ is the president of the British Society for Antimicrobial Chemotherapy and has received honoraria from Pfizer, Shionogi, Menarini and Tillots. SS has received honoraria from Pfizer and Gilead for educational purposes. PLW performed diagnostic evaluations and received meeting sponsorship from Associates of Cape Cod, Bruker, Dynamiker and Launch Diagnostics; speaker fees, expert advice fees and meeting sponsorship from Gilead; and speaker and expert advice fees from Pfizer and expert advice fees from F2G.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
