LEF1 enhances β-catenin transactivation through IDR-dependent liquid-liquid phase separation
- PMID: 37657935
- PMCID: PMC10474303
- DOI: 10.26508/lsa.202302118
LEF1 enhances β-catenin transactivation through IDR-dependent liquid-liquid phase separation
Abstract
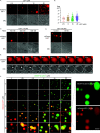
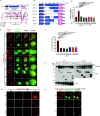
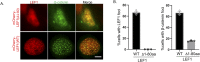
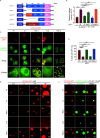
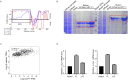
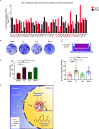
Wnt/β-catenin signaling plays a crucial role in cancer development, primarily activated by β-catenin forming a transcription complex with LEF/TCF in the nucleus and initiating the transcription of Wnt target genes. Here, we report that LEF1, a member of the LEF/TCF family, can form intrinsically disordered region (IDR)-dependent condensates with β-catenin both in vivo and in vitro, which is required for β-catenin-dependent transcription. Notably, LEF1 with disrupted IDR lost its promoting activity on tumor proliferation and metastasis, which can be restored by substituting with FUS IDR. Our findings provide new insight into the essential role of liquid-liquid phase separation in Wnt/β-catenin signaling and present a potential new target for cancer therapy.
© 2023 Zhao et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Wnt target gene activation requires β-catenin separation into biomolecular condensates.PLoS Biol. 2024 Sep 24;22(9):e3002368. doi: 10.1371/journal.pbio.3002368. eCollection 2024 Sep. PLoS Biol. 2024. PMID: 39316611 Free PMC article.
-
β-Catenin-independent activation of TCF1/LEF1 in human hematopoietic tumor cells through interaction with ATF2 transcription factors.PLoS Genet. 2013;9(8):e1003603. doi: 10.1371/journal.pgen.1003603. Epub 2013 Aug 15. PLoS Genet. 2013. PMID: 23966864 Free PMC article.
-
The transcription factor Lef1 switches partners from β-catenin to Smad3 during muscle stem cell quiescence.Sci Signal. 2018 Jul 24;11(540):eaan3000. doi: 10.1126/scisignal.aan3000. Sci Signal. 2018. PMID: 30042129
-
Discovery of chemical probes that suppress Wnt/β-catenin signaling through high-throughput screening.Cancer Sci. 2020 Mar;111(3):783-794. doi: 10.1111/cas.14297. Epub 2020 Feb 5. Cancer Sci. 2020. PMID: 31912579 Free PMC article. Review.
-
The Wnt Transcriptional Switch: TLE Removal or Inactivation?Bioessays. 2018 Feb;40(2):10.1002/bies.201700162. doi: 10.1002/bies.201700162. Epub 2017 Dec 18. Bioessays. 2018. PMID: 29250807 Free PMC article. Review.
Cited by
-
Wnt target gene activation requires β-catenin separation into biomolecular condensates.PLoS Biol. 2024 Sep 24;22(9):e3002368. doi: 10.1371/journal.pbio.3002368. eCollection 2024 Sep. PLoS Biol. 2024. PMID: 39316611 Free PMC article.
-
Wnt signaling in cancer: from biomarkers to targeted therapies and clinical translation.Mol Cancer. 2025 Apr 2;24(1):107. doi: 10.1186/s12943-025-02306-w. Mol Cancer. 2025. PMID: 40170063 Free PMC article. Review.
-
Emerging regulatory mechanisms and functions of biomolecular condensates: implications for therapeutic targets.Signal Transduct Target Ther. 2025 Jan 6;10(1):4. doi: 10.1038/s41392-024-02070-1. Signal Transduct Target Ther. 2025. PMID: 39757214 Free PMC article. Review.
-
The cancer-associated fibroblast facilitates YAP liquid-liquid phase separation to promote cancer cell stemness in HCC.Cell Commun Signal. 2025 May 24;23(1):238. doi: 10.1186/s12964-025-02256-2. Cell Commun Signal. 2025. PMID: 40413530 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
