Association of Plasma Phosphorylated Tau With the Response to Neflamapimod Treatment in Patients With Dementia With Lewy Bodies
- PMID: 37657939
- PMCID: PMC10624490
- DOI: 10.1212/WNL.0000000000207755
Association of Plasma Phosphorylated Tau With the Response to Neflamapimod Treatment in Patients With Dementia With Lewy Bodies
Abstract
Background and objectives: In a proportion of patients, dementia with Lewy bodies (DLB) is associated with Alzheimer disease (AD) copathology, which is linked to accelerated cognitive decline and more extensive cortical atrophy. The objective was to evaluate the relationship between a biomarker of AD copathology, plasma tau phosphorylated at residue 181 (ptau181), and the treatment effects of the p38α kinase inhibitor neflamapimod, which targets the cholinergic degenerative process in DLB.
Methods: The AscenD-LB study was a phase 2a, randomized (1:1), 16-week, placebo-controlled clinical trial of neflamapimod in DLB, the main results of which have been published. After the study was completed (i.e., post hoc), pretreatment plasma ptau181 levels were determined and participants were grouped based on a cutoff for AD pathology of 2.2 pg/mL (established in a separate cohort to identify AD from healthy controls). Clinical outcomes for the comparison of placebo with neflamapimod 40 mg three times daily (TID; the higher and more clinically active of 2 doses studied) were analyzed using mixed models for repeated measures within each subgroup (baseline plasma ptau181 < and ≥2.2 pg/mL).
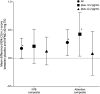
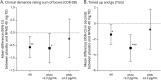
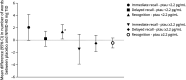
Results: Pretreatment plasma ptau181 levels were determined in eighty-five participants with mild-to-moderate DLB receiving cholinesterase inhibitors, with 45 participants below and 40 above the 2.2 pg/mL cutoff at baseline. In the 16-week treatment period, in the comparison of placebo with neflamapimod 40 mg TID, for all end points evaluated, improvements with neflamapimod treatment were greater in participants below the cutoff, compared with those above the cutoff. In addition, participants below the ptau181 cutoff at baseline showed significant improvement over placebo in an attention composite measure (+0.42, 95% CI 0.07-0.78, p = 0.023, d = 0.78), the Clinical Dementia Rating Scale Sum of Boxes (-0.60, 95% CI -1.04 to -0.06, p = 0.031, d = 0.70), the Timed Up and Go test (-3.1 seconds, 95% CI -4.7 to -1.6, p < 0.001, d = 0.74), and International Shopping List Test-Recognition (+1.4, 95% CI 0.2-2.5, p = 0.024, d = 1.00).
Discussion: Exclusion of patients with elevated plasma ptau181, potentially through excluding patients with extensive cortical neurodegeneration, enriches for a patient with DLB population that is more responsive to neflamapimod. More generally, plasma biomarkers of AD copathology at study entry should be considered as stratification variables in DLB clinical trials.
Trial registration information: NCT04001517 at ClinicalTrials.gov.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
J. Alam and J. Conway are employees of, and S.R. Doctrow is an independent contractor to, EIP Pharma Inc., a private enterprise that is developing neflamapimod and is the sponsor of the study that is the subject of this manuscript. H.-M. Chu is an employee of Anoixis, a private enterprise contracted by EIP Pharma Inc. conduct the statistical analyses. P. Maruff and S. Gomperts report no disclosures relevant to the manuscript. C. Teunissen has a collaboration contract with Quanterix Corporation, the company that provides and markets the ptau181 assay utilized in the current study. Go to
Figures
References
-
- Watson R, O'Brien JT. Differentiating dementia with Lewy bodies and Alzheimer's disease using MRI. Neurodegen Dis Manage. 2012;2(4):411-420. doi: 10.2217/nmt.12.41 - DOI
