A molecular analysis of substituted phenylethylamines as potential microtubule targeting agents through in silico methods and in vitro microtubule-polymerization activity
- PMID: 37658096
- PMCID: PMC10474033
- DOI: 10.1038/s41598-023-41600-9
A molecular analysis of substituted phenylethylamines as potential microtubule targeting agents through in silico methods and in vitro microtubule-polymerization activity
Abstract
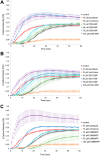
Natural phenethylamines are trace amine neurotransmitters associated with dopamine transmission and related illnesses such Parkinson's disease, and addiction. Synthetic phenethylamines can have psychoactive and hallucinogenic effects due to their high affinity with the 5-HT2A receptor. Evidence indicates phenethylamines can directly alter the microtubule cytoskeleton being structurally similar to the microtubule destabilizing agent colchicine, however little work has been done on this interaction. As microtubules provide neuron structure, intracellular transport, and influence synaptic plasticity the interaction of phenethylamines with microtubules is important for understanding the potential harms, or potential pharmaceutical use of phenethylamines. We investigated 110 phenethylamines and their interaction with microtubules. Here we performed molecular docking of these compounds at the colchicine binding site and ranked them via binding energy. The top 10% of phenethylamines were further screened based on pharmacokinetic and physicochemical properties derived from SwissADME and LightBBB. Based on these properties 25B-NBF, 25C-NBF, and DMBMPP were tested in in vitro microtubule polymerization assays showing that they alter microtubule polymerization dynamics in a dose dependent manner. As these compounds can rapidly cross the blood brain barrier and directly affect cytoskeletal dynamics, they have the potential to modulate cytoskeletal based neural plasticity. Further investigations into these mechanisms are warranted.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 2004;90:257–271. - PubMed
-
- Nelson ME, Bryant SM, Aks SE. Emerging drugs of abuse. Dis. Mon. 2014;60:110–132. - PubMed
-
- INCB. Report of the International Narcotics Control Board (INCB) for 2018. (United Nations, 2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

