Analysis of clinical failure of anti-tau and anti-synuclein antibodies in neurodegeneration using a quantitative systems pharmacology model
- PMID: 37658103
- PMCID: PMC10474108
- DOI: 10.1038/s41598-023-41382-0
Analysis of clinical failure of anti-tau and anti-synuclein antibodies in neurodegeneration using a quantitative systems pharmacology model
Abstract
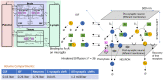
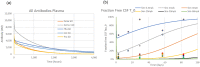
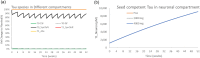
Misfolded proteins in Alzheimer's disease and Parkinson's disease follow a well-defined connectomics-based spatial progression. Several anti-tau and anti-alpha synuclein (aSyn) antibodies have failed to provide clinical benefit in clinical trials despite substantial target engagement in the experimentally accessible cerebrospinal fluid (CSF). The proposed mechanism of action is reducing neuronal uptake of oligomeric protein from the synaptic cleft. We built a quantitative systems pharmacology (QSP) model to quantitatively simulate intrasynaptic secretion, diffusion and antibody capture in the synaptic cleft, postsynaptic membrane binding and internalization of monomeric and oligomeric tau and aSyn proteins. Integration with a physiologically based pharmacokinetic (PBPK) model allowed us to simulate clinical trials of anti-tau antibodies gosuranemab, tilavonemab, semorinemab, and anti-aSyn antibodies cinpanemab and prasineuzumab. Maximal target engagement for monomeric tau was simulated as 45% (semorinemab) to 99% (gosuranemab) in CSF, 30% to 99% in ISF but only 1% to 3% in the synaptic cleft, leading to a reduction of less than 1% in uptake of oligomeric tau. Simulations for prasineuzumab and cinpanemab suggest target engagement of free monomeric aSyn of only 6-8% in CSF, 4-6% and 1-2% in the ISF and synaptic cleft, while maximal target engagement of aggregated aSyn was predicted to reach 99% and 80% in the synaptic cleft with similar effects on neuronal uptake. The study generates optimal values of selectivity, sensitivity and PK profiles for antibodies. The study identifies a gradient of decreasing target engagement from CSF to the synaptic cleft as a key driver of efficacy, quantitatively identifies various improvements for drug design and emphasizes the need for QSP modelling to support the development of tau and aSyn antibodies.
© 2023. Springer Nature Limited.
Conflict of interest statement
JPC is an employee and stock owner of discoveric bio alpha ltd. SB was an employee of Certara during this project. MW is an employee of Certara. PvdG is an employee and stock owner of Certara. HG is an employee and stock owner of Certara.
Figures




Similar articles
-
Semorinemab Pharmacokinetics and The Effect on Plasma Total Tau Pharmacodynamics in Clinical Studies.J Prev Alzheimers Dis. 2024;11(5):1241-1250. doi: 10.14283/jpad.2024.146. J Prev Alzheimers Dis. 2024. PMID: 39350369 Clinical Trial.
-
Acetylation as a major determinant to microtubule-dependent autophagy: Relevance to Alzheimer's and Parkinson disease pathology.Biochim Biophys Acta Mol Basis Dis. 2019 Aug 1;1865(8):2008-2023. doi: 10.1016/j.bbadis.2018.11.014. Epub 2018 Dec 17. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30572013
-
Effects of pharmacological modulators of α-synuclein and tau aggregation and internalization.Sci Rep. 2020 Jul 30;10(1):12827. doi: 10.1038/s41598-020-69744-y. Sci Rep. 2020. PMID: 32732936 Free PMC article.
-
Passive tau-based immunotherapy for tauopathies.Handb Clin Neurol. 2023;196:611-619. doi: 10.1016/B978-0-323-98817-9.00029-6. Handb Clin Neurol. 2023. PMID: 37620094 Review.
-
Neuropathological and Biomarker Findings in Parkinson's Disease and Alzheimer's Disease: From Protein Aggregates to Synaptic Dysfunction.J Parkinsons Dis. 2021;11(1):107-121. doi: 10.3233/JPD-202323. J Parkinsons Dis. 2021. PMID: 33325398 Free PMC article. Review.
Cited by
-
Transcytosis-Driven Treatment of Neurodegenerative Disorders by mRNA-Expressed Antibody-Transferrin Conjugates.Biomedicines. 2024 Apr 12;12(4):851. doi: 10.3390/biomedicines12040851. Biomedicines. 2024. PMID: 38672205 Free PMC article. Review.
-
Interactions of Therapeutic Antibodies With Presynaptically-Released Misfolded Proteins in Neurodegenerative Diseases. A Spatial Monte-Carlo Simulation Study.CPT Pharmacometrics Syst Pharmacol. 2025 Jul;14(7):1168-1178. doi: 10.1002/psp4.70035. Epub 2025 Apr 28. CPT Pharmacometrics Syst Pharmacol. 2025. PMID: 40296445 Free PMC article.
-
Quantitative systems pharmacology model of α-synuclein pathology in Parkinson's disease-like mouse for investigation of passive immunotherapy mechanisms.CPT Pharmacometrics Syst Pharmacol. 2024 Oct;13(10):1798-1809. doi: 10.1002/psp4.13223. Epub 2024 Aug 23. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 39177164 Free PMC article.
-
A mechanistic model of pure and lipidic α-synuclein aggregation for advancing Parkinson's therapies.Commun Chem. 2025 Jun 14;8(1):186. doi: 10.1038/s42004-025-01558-3. Commun Chem. 2025. PMID: 40517155 Free PMC article.
-
Pathological mechanisms and treatment progression of Alzheimer's disease.Eur J Med Res. 2025 Jul 14;30(1):625. doi: 10.1186/s40001-025-02886-9. Eur J Med Res. 2025. PMID: 40660381 Free PMC article. Review.
References
-
- Ossenkoppele R, Smith R, Mattsson-Carlgren N, Groot C, Leuzy A, Strandberg O, et al. Accuracy of tau positron emission tomography as a prognostic marker in preclinical and prodromal Alzheimer disease: A head-to-head comparison against amyloid positron emission tomography and magnetic resonance imaging. JAMA Neurol. 2021;78:961–971. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

