Non-chemotherapy adjuvant agents in TP53 mutant Ewing sarcoma
- PMID: 37658148
- PMCID: PMC10474113
- DOI: 10.1038/s41598-023-40751-z
Non-chemotherapy adjuvant agents in TP53 mutant Ewing sarcoma
Abstract
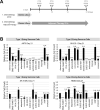
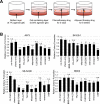
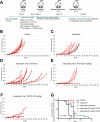
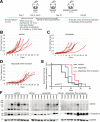
Ewing sarcoma (EWS) is a malignant tumor arising in bone or soft tissue that occurs in adolescent and young adult patients as well as adults later in life. Although non-metastatic EWS is typically responsive to treatment when newly diagnosed, relapsed cases have an unmet need for which no standard treatment approach exists. Recent phase III clinical trials for EWS comparing 7 vs 5 chemotherapy drugs have failed to improve survival. To extend the durability of remission for EWS, we investigated 3 non-chemotherapy adjuvant therapy drug candidates to be combined with chemotherapy. The efficacy of these adjuvant drugs was investigated via anchorage-dependent growth assays, anchorage-independent soft-agar colony formation assays and EWS xenograft mouse models. Enoxacin and entinostat were the most effective adjuvant drug in both long-term in vitro and in vivo adjuvant studies. In the context that enoxacin is an FDA-approved antibiotic, and that entinostat is an investigational agent not yet FDA-approved, we propose enoxacin as an adjuvant drug for further preclinical and clinical investigation in EWS patients.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
The authors declare no competing interests.
Figures

References
-
- Cornille H, Delepine NA, Alkhallaf S, Delepine G. Very late recurrence of Ewing sarcoma (ES): Report on three cases and review of literature. J. Clin. Oncol. 2011;29:e20005–e20005. doi: 10.1200/jco.2011.29.15_suppl.e20005. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

