CD31 defines a subpopulation of human adipose-derived regenerative cells with potent angiogenic effects
- PMID: 37658225
- PMCID: PMC10474028
- DOI: 10.1038/s41598-023-41535-1
CD31 defines a subpopulation of human adipose-derived regenerative cells with potent angiogenic effects
Abstract
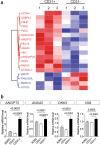
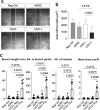
Cellular heterogeneity represents a major challenge for regenerative treatment using freshly isolated Adipose Derived Regenerative Cells (ADRCs). Emerging data suggest superior efficacy of ADRCs as compared to the ex vivo expanded and more homogeneous ADRCs (= ASCs) for indications involving (micro)vascular deficiency, however, it remains unknown which ADRC cell subtypes account for the improvement. Surprisingly, we found regarding erectile dysfunction (ED) that the number of injected CD31+ ADRCs correlated positively with erectile function 12 months after one bolus of autologous ADRCs. Comprehensive in vitro and ex vivo analyses confirmed superior pro-angiogenic and paracrine effects of human CD31+ enriched ADRCs compared to the corresponding CD31- and parent ADRCs. When CD31+, CD31- and ADRCs were co-cultured in aortic ring- and corpus cavernous tube formation assays, the CD31+ ADRCs induced significantly higher tube development. This effect was corroborated using conditioned medium (CM), while quantitative mass spectrometric analysis suggested that this is likely explained by secretory pro-angiogenic proteins including DKK3, ANGPT2, ANAX2 and VIM, all enriched in CD31+ ADRC CM. Single-cell RNA sequencing showed that transcripts of the upregulated and secreted proteins were present in 9 endothelial ADRC subsets including endothelial progenitor cells in the heterogenous non-cultured ADRCs. Our data suggest that the vascular benefit of using ADRCs in regenerative medicine is dictated by CD31+ ADRCs.
© 2023. Springer Nature Limited.
Conflict of interest statement
SPS is the CEO and owner of Blue Cell Therapeutics, Copenhagen, Denmark. The rest of the authors declare no competing interests.
Figures





References
-
- Haahr MK, et al. A 12-month follow-up after a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: An open-label phase I clinical trial. Urology. 2018;121(203):e203–e206. - PubMed
-
- Serrero M, et al. Long-term safety and efficacy of local microinjection combining autologous microfat and adipose-derived stromal vascular fraction for the treatment of refractory perianal fistula in crohn’s disease. Gastroenterology. 2019;156:2335–2337. - PubMed
-
- Sørensen KM, Jensen CH, Sheikh SP, Qvist N, Sørensen JA. Treatment of fistulizing perianal crohn’s disease by autologous microfat enriched with adipose-derived regenerative cells. Inflamm. Bowel Dis. 2021;28:967–970. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

