The Predictive Utility of MammaPrint and BluePrint in Identifying Patients with Locally Advanced Breast Cancer Who are Most Likely to Have Nodal Downstaging and a Pathologic Complete Response After Neoadjuvant Chemotherapy
- PMID: 37658272
- PMCID: PMC10625953
- DOI: 10.1245/s10434-023-14027-9
The Predictive Utility of MammaPrint and BluePrint in Identifying Patients with Locally Advanced Breast Cancer Who are Most Likely to Have Nodal Downstaging and a Pathologic Complete Response After Neoadjuvant Chemotherapy
Abstract
Background: Neoadjuvant chemotherapy (NCT) increases the feasibility of surgical resection by downstaging large primary breast tumors and nodal involvement, which may result in surgical de-escalation and improved outcomes. This subanalysis from the Multi-Institutional Neo-adjuvant Therapy MammaPrint Project I (MINT) trial evaluated the association between MammaPrint and BluePrint with nodal downstaging.
Patients and methods: The prospective MINT trial (NCT01501487) enrolled 387 patients between 2011 and 2016 aged ≥ 18 years with invasive breast cancer (T2-T4). This subanalysis includes 146 patients with stage II-III, lymph node positive, who received NCT. MammaPrint stratifies tumors as having a Low Risk or High Risk of distant metastasis. Together with MammaPrint, BluePrint genomically (g) categorizes tumors as gLuminal A, gLuminal B, gHER2, or gBasal.
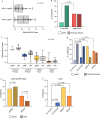
Results: Overall, 45.2% (n = 66/146) of patients had complete nodal downstaging, of whom 60.6% (n = 40/66) achieved a pathologic complete response. MammaPrint and combined MammaPrint and BluePrint were significantly associated with nodal downstaging (p = 0.007 and p < 0.001, respectively). A greater proportion of patients with MammaPrint High Risk tumors had nodal downstaging compared with Low Risk (p = 0.007). When classified with MammaPrint and BluePrint, more patients with gLuminal B, gHER2, and gBasal tumors had nodal downstaging compared with HR+HER2-, gLuminal A tumors (p = 0.538, p < 0.001, and p = 0.013, respectively).
Conclusions: Patients with genomically High Risk tumors, defined by MammaPrint with or without BluePrint, respond better to NCT and have a higher likelihood of nodal downstaging compared with patients with gLuminal A tumors. These genomic signatures can be used to select node-positive patients who are more likely to have nodal downstaging and avoid invasive surgical procedures.
Keywords: 70-gene signature; 80-gene signature; Axillary staging; Nodal downstaging; Surgical management.
© 2023. The Author(s).
Figures


References
-
- Fayanju OM, Ren Y, Thomas SM, et al. The clinical significance of breast-only and node-only pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT): a review of 20,000 breast cancer patients in the National Cancer Data Base (NCDB) Ann Surg. 2018;268(4):591–601. doi: 10.1097/SLA.0000000000002953. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

