Composite endpoints, including patient reported outcomes, in rare diseases
- PMID: 37658423
- PMCID: PMC10474650
- DOI: 10.1186/s13023-023-02819-x
Composite endpoints, including patient reported outcomes, in rare diseases
Abstract
Background: When assessing the efficacy of a treatment in any clinical trial, it is recommended by the International Conference on Harmonisation to select a single meaningful endpoint. However, a single endpoint is often not sufficient to reflect the full clinical benefit of a treatment in multifaceted diseases, which is often the case in rare diseases. Therefore, the use of a combination of several clinically meaningful outcomes is preferred. Many methodologies that allow for combining outcomes in a so-called composite endpoint are however limited in a number of ways, not in the least in the number and type of outcomes that can be combined and in the poor small-sample properties. Moreover, patient reported outcomes, such as quality of life, often cannot be integrated in a composite analysis, in spite of their intrinsic value.
Results: Recently, a class of non-parametric generalized pairwise comparisons tests have been proposed, which members do allow for any number and type of outcomes, including patient reported outcomes. The class enjoys good small-sample properties. Moreover, this very flexible class of methods allows for prioritizing the outcomes by clinical severity, allows for matched designs and for adding a threshold of clinical relevance. Our aim is to introduce the generalized pairwise comparison ideas and concepts for rare disease clinical trial analysis, and demonstrate their benefit in a post-hoc analysis of a small-sample trial in epidermolysis bullosa. More precisely, we will include a patient relevant outcome (Quality of life), in a composite endpoint. This publication is part of the European Joint Programme on Rare Diseases (EJP RD) series on innovative methodologies for rare diseases clinical trials, which is based on the webinars presented within the educational activity of EJP RD. This publication covers the webinar topic on composite endpoints in rare diseases and includes participants' response to a questionnaire on this topic.
Conclusions: Generalized pairwise comparisons is a promising statistical methodology for evaluating any type of composite endpoints in rare disease trials and may allow a better evaluation of therapy efficacy including patients reported outcomes in addition to outcomes related to the diseases signs and symptoms.
Keywords: Composite endpoints; EJP-RD; Epidermolysis bullosa; Generalized pairwise comparisons; Patient reported outcomes; Quality of life; Rare disease.
© 2023. Institut National de la Santé et de la Recherche Médicale (INSERM).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

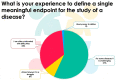



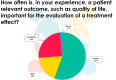
References
-
- Wally V, Hovnanian A, Ly J, Buckova H, Brunner V, Lettner T, et al. Diacerein orphan drug development for epidermolysis bullosa simplex: a phase 2/3 randomized, placebo-controlled, double-blind clinical trial. J Am Acad Dermatol. 2018;78(5):892–901. - PubMed
-
- Barnard GA. Significance tests for 2 × 2 tables. Biometrika. 1947;34:123–38. - PubMed
-
- European Medicines Agency Committee For Human Medicinal Products (CHMP). Guideline on multiplicity issues in clinical trials. 2017; https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guidel... Accessed 25 April 2022.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

