Chemogenetic activation of locus coeruleus neurons ameliorates the severity of multiple sclerosis
- PMID: 37658434
- PMCID: PMC10474779
- DOI: 10.1186/s12974-023-02865-z
Chemogenetic activation of locus coeruleus neurons ameliorates the severity of multiple sclerosis
Abstract
Background: Most current disease-modifying therapies approved for multiple sclerosis (MS) are immunomodulatory drugs that counteract the aberrant activity of the immune system. Hence, new pharmacological interventions that drive anti-inflammatory activity and neuroprotection would represent interesting alternative therapeutic approaches or complementary strategies to treat progressive forms of MS. There is evidence of reduced noradrenaline levels and alterations to locus coeruleus (LC) noradrenergic neurons in MS patients, as well as in animal models of this disease, potentially factors contributing to the pathophysiology. Drugs that enhance noradrenaline appear to have some beneficial effects in MS, suggesting their potential to dampen the underlying pathology and disease progression.
Methods: Therefore, we explored the consequences of chronic LC noradrenergic neurons activation by chemogenetics in experimental autoimmune encephalomyelitis (EAE) mice, the most widely used experimental model of MS. LC activation from the onset or the peak of motor symptoms was explored as two different therapeutic approaches, assessing the motor and non-motor behavioral changes as EAE progresses, and studying demyelination, inflammation and glial activation in the spinal cord and cerebral cortex during the chronic phase of EAE.
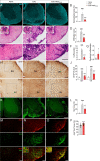
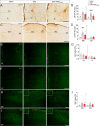
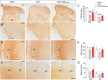
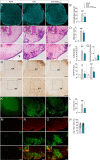
Results: LC activation from the onset of motor symptoms markedly alleviated the motor deficits in EAE mice, as well as their anxiety-like behavior and sickness, in conjunction with reduced demyelination and perivascular infiltration in the spinal cord and glial activation in the spinal cord and prefrontal cortex (PFC). When animals exhibited severe paralysis, LC activation produced a modest alleviation of EAE motor symptoms and it enhanced animal well-being, in association with an improvement of the EAE pathology at the spinal cord and PFC level. Interestingly, the reduced dopamine beta-hydroxylase expression associated with EAE in the spinal cord and PFC was reversed through chemogenetic LC activation.
Conclusion: Therefore, clear anti-inflammatory and neuroprotective effects were produced by the selective activation of LC noradrenergic neurons in EAE mice, having greater benefits when LC activation commenced earlier. Overall, these data suggest noradrenergic LC neurons may be targets to potentially alleviate some of the motor and non-motor symptoms in MS.
Keywords: Experimental autoimmune encephalomyelitis; Locus coeruleus; Multiple sclerosis; Noradrenaline; Spinal cord.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis.Brain. 2011 Mar;134(Pt 3):665-77. doi: 10.1093/brain/awq362. Epub 2011 Feb 4. Brain. 2011. PMID: 21297130 Free PMC article.
-
Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system.J Neurochem. 2016 Oct;139 Suppl 2:154-178. doi: 10.1111/jnc.13447. Epub 2016 Mar 10. J Neurochem. 2016. PMID: 26968403 Review.
-
The vincamine derivative vindeburnol provides benefit in a mouse model of multiple sclerosis: effects on the Locus coeruleus.J Neurochem. 2012 Apr;121(2):206-16. doi: 10.1111/j.1471-4159.2012.07673.x. Epub 2012 Feb 17. J Neurochem. 2012. PMID: 22288774
-
Role of catalpol in ameliorating the pathogenesis of experimental autoimmune encephalomyelitis by increasing the level of noradrenaline in the locus coeruleus.Mol Med Rep. 2018 Mar;17(3):4163-4172. doi: 10.3892/mmr.2018.8378. Epub 2018 Jan 5. Mol Med Rep. 2018. PMID: 29328415 Free PMC article.
-
The Emerging Role of Zinc in the Pathogenesis of Multiple Sclerosis.Int J Mol Sci. 2017 Sep 28;18(10):2070. doi: 10.3390/ijms18102070. Int J Mol Sci. 2017. PMID: 28956834 Free PMC article. Review.
Cited by
-
ANA-12 Targets and Inhibits BDNF/TrkB Signaling to Alleviate Pain Behaviors in Rheumatoid Arthritis Mice.Neurochem Res. 2025 Jul 17;50(4):234. doi: 10.1007/s11064-025-04487-8. Neurochem Res. 2025. PMID: 40673956 Free PMC article.
-
Regenerating Locus Coeruleus-Norepinephrine (LC-NE) Function: A Novel Approach for Neurodegenerative Diseases.Cell Prolif. 2025 Jun;58(6):e13807. doi: 10.1111/cpr.13807. Epub 2025 Jan 28. Cell Prolif. 2025. PMID: 39876531 Free PMC article. Review.
References
-
- The Multiple Sclerosis International Federation. Atlas of MS. 3rd ed. 2020.
-
- McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325(8):765–779. - PubMed
MeSH terms
Substances
Grants and funding
- #FPU20-03072/"Agencia Estatal de Investigación-Ministerio de Ciencia, Innovación y Universidades"; FPU fellowship
- PID2022-1427850B-I00/"Fondo Europeo de Desarrollo Regional" (FEDER)-UE "A way to build Europe" from the "Ministerio de Economía y Competitividad
- PDC2022-133987-I00/"Fondo Europeo de Desarrollo Regional" (FEDER)-UE "A way to build Europe" from the "Ministerio de Economía y Competitividad
- PY20_00958/"Consejería de Transformación Económica, Industria, Conocimiento y Universidad, Junta de Andalucía"
- CTS-510/"Consejería de Transformación Económica, Industria, Conocimiento y Universidad, Junta de Andalucía"
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

