Effectiveness and safety of mesenchymal stem/stromal cell for radiation-induced hyposalivation and xerostomia in previous head and neck cancer patients (MESRIX-III): a study protocol for a single-centre, double-blinded, randomised, placebo-controlled, phase II study
- PMID: 37658468
- PMCID: PMC10474624
- DOI: 10.1186/s13063-023-07594-5
Effectiveness and safety of mesenchymal stem/stromal cell for radiation-induced hyposalivation and xerostomia in previous head and neck cancer patients (MESRIX-III): a study protocol for a single-centre, double-blinded, randomised, placebo-controlled, phase II study
Abstract
Background: A predominant side effect of radiotherapy for head and neck cancer is salivary gland hypofunction and xerostomia leading to debilitating oral disorders and impaired quality of life (QoL). Intraglandular mesenchymal stem cell therapy has shown promising results as a treatment for xerostomia.
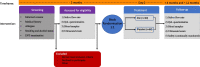
Methods: This is a randomised, double-blinded, placebo-controlled, parallel-group, prospective, single-centre trial investigating the safety, tolerability, and effectiveness of allogeneic stem cells as a treatment for radiation-induced hyposalivation and xerostomia for previous head and neck cancer patients. We will include a total of 120 patients who previously have been treated with radiotherapy for a head and neck cancer in Denmark. Participants will be randomly assigned using block randomisation to one of two parallel groups in a 1:1 ratio to receive ultrasound-guided injection of allogeneic adipose-derived mesenchymal stem cell (ASC) (n = 60) or placebo (n = 60) into the submandibular glands. Placebo will consist of CryoStor10 (BiolifeSolutions), the freeze media for ASCs containing 10% dimethyl sulfoxide (DMSO). The primary endpoint is change in unstimulated whole saliva flow rate. The secondary endpoints are change in stimulated whole saliva flow rate, QoL, and composition of saliva. Further secondary endpoints are safety and immune response (human leukocyte antigen (HLA) response) to the stem cells will be assessed. Patients are evaluated at baseline (before treatment), after 4 months, and after 12 months. All study personnel, except study personnel thawing and preparing the treatment for injection, and participants will be blinded to group assignment. Unblinded study personnel will not participate in the outcome assessment.
Discussion: The trials will investigate the efficacy and safety of ASC injection to the submandibular gland as a potential new treatment for post-radiation xerostomia. We hope the results will pave the way for a clinically relevant treatment to ameliorate patients with xerostomia, a severely hampering condition.
Trial registration: The study is approved by the Danish Data Protection Agency (protocol number P-2020-1164), the National Ethics Committee protocol number: (Protocol number: 1802872), and the Danish Medical Agency (2018-000348-24). The protocol was registered at the ClinicalTrials.gov database (NCT04776538).
Keywords: Hyposalivation; MSC; Mesenchymal stem cells; Mesenchymal stromal cells; Xerostomia.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors C.D.L., C.G., and C.V.B. declare the following competing interests: Rigshospitalet and the University of Copenhagen have a pending patent application entitled “Stem cell therapy for salivary gland dysfunction” published as WO 2020/165405 A1, with K.K.J, C.D.L., C.G., and C.V.B. as the inventors. The authors A.E., M.H.S. and J.K. declare the following competing interests: A.E., M.H.S. and J.K. are inventors of the patent “Stem cell therapy based on adipose-derived stem cells” (Publication WO 2017-068140). All authors met the ICMJE authorship criteria. No honoraria or payments were made for authorship. The remaining authors declare no competing interests.
Figures
Similar articles
-
First-in-man mesenchymal stem cells for radiation-induced xerostomia (MESRIX): study protocol for a randomized controlled trial.Trials. 2017 Mar 7;18(1):108. doi: 10.1186/s13063-017-1856-0. Trials. 2017. PMID: 28270226 Free PMC article. Clinical Trial.
-
Long-term Effectiveness and Safety of Mesenchymal Stromal Cell Therapy for Radiation-Induced Hyposalivation in Head and Neck Cancer Survivors: A Randomized Phase II Trial.Clin Cancer Res. 2025 Mar 3;31(5):824-831. doi: 10.1158/1078-0432.CCR-24-2663. Clin Cancer Res. 2025. PMID: 39751638 Clinical Trial.
-
Mesenchymal Stem/Stromal Cell Therapy for Radiation-Induced Xerostomia in Previous Head and Neck Cancer Patients: A Phase II Randomized, Placebo-Controlled Trial.Clin Cancer Res. 2024 May 15;30(10):2078-2084. doi: 10.1158/1078-0432.CCR-23-3675. Clin Cancer Res. 2024. PMID: 38441659 Free PMC article. Clinical Trial.
-
Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers.Int J Radiat Oncol Biol Phys. 2010 Nov 15;78(4):983-91. doi: 10.1016/j.ijrobp.2010.06.052. Int J Radiat Oncol Biol Phys. 2010. PMID: 20970030 Free PMC article. Review.
-
Salivary Gland Hypofunction and Xerostomia in Head and Neck Radiation Patients.J Natl Cancer Inst Monogr. 2019 Aug 1;2019(53):lgz016. doi: 10.1093/jncimonographs/lgz016. J Natl Cancer Inst Monogr. 2019. PMID: 31425600 Review.
Cited by
-
Role of Mesenchymal Stem/Stromal Cells in Head and Neck Cancer-Regulatory Mechanisms of Tumorigenic and Immune Activity, Chemotherapy Resistance, and Therapeutic Benefits of Stromal Cell-Based Pharmacological Strategies.Cells. 2024 Jul 28;13(15):1270. doi: 10.3390/cells13151270. Cells. 2024. PMID: 39120301 Free PMC article. Review.
-
Cancer therapy-related salivary dysfunction.J Clin Invest. 2024 Sep 3;134(17):e182661. doi: 10.1172/JCI182661. J Clin Invest. 2024. PMID: 39225092 Free PMC article. Review.
-
CD9-enriched extracellular vesicles from chemically reprogrammed basal progenitors of salivary glands mitigate salivary gland fibrosis.Bioact Mater. 2025 Jan 24;47:229-247. doi: 10.1016/j.bioactmat.2025.01.019. eCollection 2025 May. Bioact Mater. 2025. PMID: 39925710 Free PMC article.
-
Development of human salivary gland cell lines for modeling radiation-induced damage in three-dimensional spheroid cultures.J Tissue Eng. 2025 Apr 30;16:20417314251326667. doi: 10.1177/20417314251326667. eCollection 2025 Jan-Dec. J Tissue Eng. 2025. PMID: 40322739 Free PMC article.
-
A review of adipose-derived mesenchymal stem cells' impacts and challenges: metabolic regulation, tumor modulation, immunomodulation, regenerative medicine and genetic engineering therapies.Front Endocrinol (Lausanne). 2025 May 29;16:1606847. doi: 10.3389/fendo.2025.1606847. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40510466 Free PMC article. Review.
References
-
- Borras JM, Barton M, Grau C, Corral J, Verhoeven R, Lemmens V, et al. The impact of cancer incidence and stage on optimal utilization of radiotherapy: methodology of a population based analysis by the ESTRO-HERO project. Radiother Oncol. 2015;116:45–50. doi: 10.1016/j.radonc.2015.04.021. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous