A fully interpretable machine learning model for increasing the effectiveness of urine screening
- PMID: 37658807
- PMCID: PMC10691191
- DOI: 10.1093/ajcp/aqad099
A fully interpretable machine learning model for increasing the effectiveness of urine screening
Abstract
Objectives: This article addresses the need for effective screening methods to identify negative urine samples before urine culture, reducing the workload, cost, and release time of results in the microbiology laboratory. We try to overcome the limitations of current solutions, which are either too simple, limiting effectiveness (1 or 2 parameters), or too complex, limiting interpretation, trust, and real-world implementation ("black box" machine learning models).
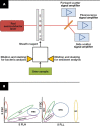
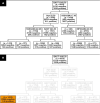
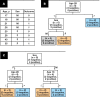
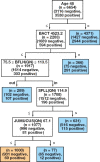
Methods: The study analyzed 15,312 samples from 10,534 patients with clinical features and the Sysmex Uf-1000i automated analyzer data. Decision tree (DT) models with or without lookahead strategy were used, as they offer a transparent set of logical rules that can be easily understood by medical professionals and implemented into automated analyzers.
Results: The best model achieved a sensitivity of 94.5% and classified negative samples based on age, bacteria, mucus, and 2 scattering parameters. The model reduced the workload by an additional 16% compared to the current procedure in the laboratory, with an estimated financial impact of €40,000/y considering 15,000 samples/y. Identified logical rules have a scientific rationale matched to existing knowledge in the literature.
Conclusions: Overall, this study provides an effective and interpretable screening method for urine culture in microbiology laboratories, using data from the Sysmex UF-1000i automated analyzer. Unlike other machine learning models, our model is interpretable, generating trust and enabling real-world implementation.
Keywords: data science; decision tree; machine learning; urinalysis.
© American Society for Clinical Pathology, 2023.
Figures


References
-
- Boonen K, Koldewijn E, Arents N, Raaymakers P, Scharnhorst V.. Urine flow cytometry as a primary screening method to exclude urinary tract infections. World J Urol. 2013;31(3):547-551. - PubMed
-
- Enko D, Stelzer I, Böckl M, et al. Comparison of the reliability of Gram-negative and Gram-positive flags of the Sysmex UF-5000 with manual Gram stain and urine culture results. Clin Chem Lab Med. 2021;59(3):619-624. - PubMed
-
- De Rosa R, Grosso S, Bruschetta G, et al. Evaluation of the Sysmex UF1000i flow cytometer for ruling out bacterial urinary tract infection. Clin Chim Acta. 2010;411(15-16):1137-1142. - PubMed
-
- De Rosa R, Grosso S, Lorenzi G, Bruschetta G, Camporese A.. Evaluation of the new Sysmex UF-5000 fluorescence flow cytometry analyser for ruling out bacterial urinary tract infection and for prediction of Gram negative bacteria in urine cultures. Clin Chim Acta. 2018;484:171-178. 10.1016/j.cca.2018.05.047 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

