The Assessment of Patient-Reported Outcomes for the Authorisation of Medicines in Europe: A Review of European Public Assessment Reports from 2017 to 2022
- PMID: 37659000
- PMCID: PMC10627987
- DOI: 10.1007/s40258-023-00827-3
The Assessment of Patient-Reported Outcomes for the Authorisation of Medicines in Europe: A Review of European Public Assessment Reports from 2017 to 2022
Abstract
Objectives: Health regulators have progressively increased their attention and focus on patient-reported outcomes (PROs), driven by the diffusion of a patient-centred approach to the drug development process. This study investigates the consideration of PROs and their measures (PROMs) in the authorisation of medicines in Europe.
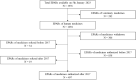
Methods: All medicines for human use authorised or refused by the European Medicines Agency (EMA) in the period 2017-2022 were identified, and corresponding European Public Assessment Reports (EPARs) were downloaded for review. Medicine and PROs/PROM characteristics were systematically recorded. A multivariate logistic regression was performed to identify variables associated with the use of patient-reported evidence in EPARs.
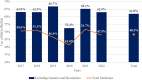
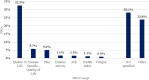
Results: Overall, 497 EPARs of authorised medicines and 19 EPARs of refused medicines were analysed; of these, 240 (48.3%) and 10 (52.6%), respectively, reported any use of PROs/PROMs (p = 0.710). For authorised medicines, the likelihood of using PROs/PROMs was negatively affected by generic (OR = 0.01, p < 0.001) and biosimilar status (OR = 0.46, p = 0.013) and positively affected by orphan status (OR = 1.41, p = 0.177). The use of PROMs (50.6% in 2017 vs 47.9% in 2022) did not show a clear pattern over the 6-year period considered (p = 0.758) and was particularly uncommon in some therapeutic areas (e.g., 15.2% in infectious diseases). A total of 816 dyads of PROs/PROMs were identified. On average each EPAR considered 1.6 (range: 0-14) instruments. Patient-reported outcomes were typically secondary (53.3%) and exploratory endpoints (18.8%); in one-third of cases (32.5%), they assessed generic quality of life. Among the PROMs, 227 (27.8%) targeted general population; EQ-5D (11.0%), SF-36/SF-12 (5.9%) and EORTC QLQ-C30 (5.6%) were the instruments most frequently used.
Conclusions: This study suggests PROs/PROMs are considered in less than half of total medicine assessments and even more rarely in some disease areas. The adoption of PROs is key in EMA strategy to 2025 and would be facilitated by consensus development on their measures and optimisation of data collection.
© 2023. The Author(s).
Conflict of interest statement
MM, FM, and OC have no conflict of interest. SA is employed at Roche S.p.A.
Figures

Comment in
-
Comment on: "The Assessment of Patient-Reported Outcomes for the Authorisation of Medicines in Europe: A Review of European Public Assessment Reports from 2017 to 2022".Appl Health Econ Health Policy. 2024 Jan;22(1):123-124. doi: 10.1007/s40258-023-00850-4. Epub 2023 Nov 16. Appl Health Econ Health Policy. 2024. PMID: 37971664 No abstract available.
References
-
- Minvielle E, Fierobe A, Fourcade A, Ferrua M, di Palma M, Scotté F, Mir O. The use of patient-reported outcome and experience measures for health policy purposes: a scoping review in oncology. Health Policy. 2023;129:104702. 10.1016/j.healthpol.2022.12.010. - PubMed
-
- European Medicines Agency (EMA). Patient experience data in EU medicines development and regulatory decision making. https://www.ema.europa.eu/en/events/multi-stakeholder-workshop-patient-e.... Accessed 7 Nov 2022.
-
- Food and Drug Administration (FDA). Clinical Outcome Assessment (COA): Frequently Asked Questions. https://www.fda.gov/about-fda/clinical-outcome-assessment-coa-frequently.... Accessed 14 June 2023.
-
- Food and Drug Administration (FDA). Guidance Document. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Guidance for Industry. December 2009. https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed 7 Nove 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

