Mycophenolate mofetil as a treatment for presumed idiopathic chronic hepatitis in dogs: Six cases (2010-2022)
- PMID: 37659075
- PMCID: PMC10650243
- DOI: 10.1002/vms3.1261
Mycophenolate mofetil as a treatment for presumed idiopathic chronic hepatitis in dogs: Six cases (2010-2022)
Abstract
Objectives: The objectives of this study were to describe the clinical findings, treatment and outcomes of six dogs with presumed idiopathic chronic hepatitis treated with mycophenolate mofetil (MMF).
Materials and methods: Medical records were retrospectively searched to identify dogs in which idiopathic chronic hepatitis was diagnosed on histopathology between January 2010 and June 2022 that were treated with MMF for at least two weeks with >2 follow-up examinations. Data recorded from each dog included signalment, clinical signs, diagnostic test results and treatment.
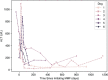
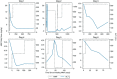
Results: Six dogs were treated with MMF at a median initial dosage of 9.6 mg/kg PO q 12 h. Reported adverse effects from MMF included decreased appetite, vomiting and diarrhoea. In all six dogs, MMF was used successfully long term for the treatment of idiopathic chronic hepatitis as determined by 46% or greater improvement of alanine aminotransferase (ALT) between 4 and 18 weeks of starting MMF. Three dogs were also temporarily treated for 4-6 months on a tapering dose of prednisone. In two dogs, ALT remained within the reference interval, and in one dog, it was very mildly elevated when on MMF alone. In all six dogs, owners reported that the medication was well tolerated.
Clinical significance: To the authors' knowledge, this is the first report describing the use of MMF with and without a tapering dose of prednisone for the treatment of idiopathic chronic hepatitis in six dogs. Based on the outcomes of the dogs in this report, MMF can be effective for the long-term treatment of idiopathic chronic hepatitis as measured by reduction in ALT and improvement of clinical signs.
Keywords: dog; hepatic disease; hepatitis; search terms.
© 2023 The Authors. Veterinary Medicine and Science published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of Interest.
Figures
References
-
- Broaddus, K. D. , Tillson, D. M. , Lenz, S. D. , Niemeyer, G. P. , Brawner, W. R. , Welch, J. A. , & Lothrop, C. D. (2006). Renal allograft histopathology in dog leukocyte antigen mismatched dogs after renal transplantation. Veterinary Surgery, 35(2), 125–135. 10.1111/j.1532-950x.2006.00123.x - DOI - PubMed
-
- Dirksen, K. , Burgener, I. A. , Rothuizen, J. , Van Den Ingh, T. S. G. A. M. , Penning, L. C. , Spee, B. , & Fieten, H. (2017). Sensitivity and specificity of plasma ALT, ALP, and bile acids for hepatitis in labrador retrievers. Journal of Veterinary Internal Medicine, 31(4), 1017–1027. 10.1111/jvim.14716 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

