Active cortical networks promote shunting fast synaptic inhibition in vivo
- PMID: 37659408
- PMCID: PMC11913778
- DOI: 10.1016/j.neuron.2023.08.005
Active cortical networks promote shunting fast synaptic inhibition in vivo
Abstract
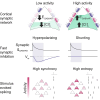
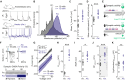
Fast synaptic inhibition determines neuronal response properties in the mammalian brain and is mediated by chloride-permeable ionotropic GABA-A receptors (GABAARs). Despite their fundamental role, it is still not known how GABAARs signal in the intact brain. Here, we use in vivo gramicidin recordings to investigate synaptic GABAAR signaling in mouse cortical pyramidal neurons under conditions that preserve native transmembrane chloride gradients. In anesthetized cortex, synaptic GABAARs exert classic hyperpolarizing effects. In contrast, GABAAR-mediated synaptic signaling in awake cortex is found to be predominantly shunting. This is due to more depolarized GABAAR equilibrium potentials (EGABAAR), which are shown to result from the high levels of synaptic activity that characterize awake cortical networks. Synaptic EGABAAR observed in awake cortex facilitates the desynchronizing effects of inhibitory inputs upon local networks, which increases the flexibility of spiking responses to external inputs. Our findings therefore suggest that GABAAR signaling adapts to optimize cortical functions.
Keywords: GABA-A receptor signaling; cortex; equilibrium potential; ionic driving force; network activity; population coupling; stimulus discrimination; synaptic inhibition.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

