Longitudinal interplay between subclinical atherosclerosis, cardiovascular risk factors, and cerebral glucose metabolism in midlife: results from the PESA prospective cohort study
- PMID: 37659430
- PMCID: PMC10469266
- DOI: 10.1016/S2666-7568(23)00134-4
Longitudinal interplay between subclinical atherosclerosis, cardiovascular risk factors, and cerebral glucose metabolism in midlife: results from the PESA prospective cohort study
Abstract
Background: Cardiovascular disease and dementia often coexist at advanced stages. Yet, longitudinal studies examining the interplay between atherosclerosis and its risk factors on brain health in midlife are scarce. We aimed to characterise the longitudinal associations between cerebral glucose metabolism, subclinical atherosclerosis, and cardiovascular risk factors in middle-aged asymptomatic individuals.
Methods: The Progression of Early Subclinical Atherosclerosis (PESA) study is a Spanish longitudinal observational cohort study of 4184 asymptomatic individuals aged 40-54 years (NCT01410318). Participants with subclinical atherosclerosis underwent longitudinal cerebral [18F]fluorodeoxyglucose ([18F]FDG)-PET, and annual percentage change in [18F]FDG uptake was assessed (primary outcome). Cardiovascular risk was quantified with SCORE2 and subclinical atherosclerosis with three-dimensional vascular ultrasound (exposures). Multivariate regression and linear mixed effects models were used to assess associations between outcomes and exposures. Additionally, blood-based biomarkers of neuropathology were quantified and mediation analyses were performed. Secondary analyses were corrected for multiple comparisons using the false discovery rate (FDR) approach.
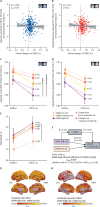
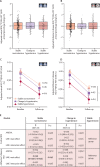
Findings: This longitudinal study included a PESA subcohort of 370 participants (median age at baseline 49·8 years [IQR 46·1-52·2]; 309 [84%] men, 61 [16%] women; median follow-up 4·7 years [IQR 4·2-5·2]). Baseline scans took place between March 6, 2013, and Jan 21, 2015, and follow-up scans between Nov 24, 2017, and Aug 7, 2019. Persistent high risk of cardiovascular disease was associated with an accelerated decline of cortical [18F]FDG uptake compared with low risk (β=-0·008 [95% CI -0·013 to -0·002]; pFDR=0·040), with plasma neurofilament light chain, a marker of neurodegeneration, mediating this association by 20% (β=0·198 [0·008 to 0·740]; pFDR=0·050). Moreover, progression of subclinical carotid atherosclerosis was associated with an additional decline in [18F]FDG uptake in Alzheimer's disease brain regions, not explained by cardiovascular risk (β=-0·269 [95% CI -0·509 to -0·027]; p=0·029).
Interpretation: Middle-aged asymptomatic individuals with persistent high risk of cardiovascular disease and subclinical carotid atherosclerosis already present brain metabolic decline, suggesting that maintenance of cardiovascular health during midlife could contribute to reductions in neurodegenerative disease burden later in life.
Funding: Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III, Santander Bank, Pro-CNIC Foundation, BrightFocus Foundation, BBVA Foundation, "la Caixa" Foundation.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests CT-P received travel grants from The Company of Biologists and International Society of Cerebral Blood Flow and Metabolism. JS-G is a Philips employee. MS has served as a consultant and at advisory boards for Roche Diagnostics International and NovoNordisk; and is a cofounder of ScanDx Sweden. MS-C has served as a consultant and at advisory boards for Roche Diagnostics International; given lectures in symposia sponsored by Roche Diagnostics and Roche Farma; and was granted with a project funded by Roche Diagnostics International. HZ has served at scientific advisory boards or as a consultant for AbbVie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche; and has served as chair of the Alzheimer's Association Global Biomarkers Standardization Consortium. KB has served at scientific advisory boards or as a consultant for Acumen, ALZpath, BioArctic, Biogen, Eisai, Lilly, Julius Clinical, Novartis, Ono Pharma, Roche Diagnostics, and Siemens Healthineers; and has produced or participated in educational programmes for Biogen, Eisai, and Roche Diagnostics. HZ and KB are cofounders of Brain Biomarker Solutions in Gothenburg, which is a part of the GU Ventures Incubator Program (unrelated to the submitted work). JDG reports research support from GE Healthcare, Roche Diagnostics, and Hoffmann-La Roche; has given lectures in symposia sponsored by General Electric, Philips, Life Molecular Imaging, and Biogen; has served on scientific advisory boards or as a consultant for Prothena and Roche Diagnostics; and is the inventor, founder, and co-owner of BetaScreen. All other authors declare no competing interests.
Figures

Comment in
-
What is good for the heart is good for brain glucose metabolism.Lancet Healthy Longev. 2023 Sep;4(9):e448-e449. doi: 10.1016/S2666-7568(23)00167-8. Lancet Healthy Longev. 2023. PMID: 37659422 No abstract available.
References
-
- Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. - PubMed
-
- Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1608–1617. - PubMed
-
- Fernández-Ortiz A, Jiménez-Borreguero LJ, Peñalvo JL, et al. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: rationale and design. Am Heart J. 2013;166:990–998. - PubMed
-
- Ibanez B, Fernández-Ortiz A, Fernández-Friera L, García-Lunar I, Andrés V, Fuster V. Progression of Early Subclinical Atherosclerosis (PESA) study: JACC Focus Seminar 7/8. J Am Coll Cardiol. 2021;78:156–179. - PubMed
-
- Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation. 2015;131:2104–2113. - PubMed

