Rheumatoid arthritis and older age are associated with lower humoral and cellular immune response to primary series COVID-19 mRNA vaccine
- PMID: 37659895
- PMCID: PMC11606055
- DOI: 10.1016/j.vaccine.2023.08.033
Rheumatoid arthritis and older age are associated with lower humoral and cellular immune response to primary series COVID-19 mRNA vaccine
Abstract
Objective: People with autoimmune disease have worse COVID-19 infection-related outcomes, lower antibody responses to COVID-19 vaccine, and higher rates of breakthrough infection. Immunosuppressive medications used to treat rheumatoid arthritis (RA) are associated with lower COVID-19 vaccine responses, though independent contributions of comorbidities, T-cell immunity, and age are less clear. We sought to test the hypothesis that RA, immunosuppressive medications used to treat RA, and older age, contribute to reduced B and T cell response to COVID-19 vaccine.
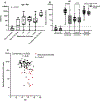
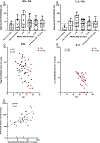
Methods: We evaluated serum samples, taken the day of 1st vaccine dose, the day of 2nd dose, 2-6 weeks after 2nd dose, 7-12 weeks after 2nd dose, 13-24 weeks after 2nd dose, and 2-6 weeks after the 3rd dose, for anti-spike IgG and neutralizing antibody levels to Wuhan and Omicron BA.1 and peripheral blood mononuclear cells (PBMC) for spike-specific IFN-γ and IL-2 production by ELISPOT assay in 46 RA and 101 non-autoimmune control participants before and after the primary series COVID-19 mRNA vaccination.
Results: RA participants had lower spike-specific IgG and Wuhan-strain neutralizing antibody levels 2-6 weeks compared to controls after the second dose of primary vaccine series. Neutralizing antibody levels against Omicron BA.1 were low in both groups. IFN-γ production correlated with Wuhan neutralizing antibody levels, while older age negatively correlated with spike-specific IL-2, IFN-γ and IgG. Lower antibody levels were associated with older age, RA status, and medication usage, while lower T cell responses were associated primarily with older age.
Conclusions: These data indicate lower COVID-19 mRNA vaccine-induced antibody levels in persons with RA compared to individuals without RA, likely partially attributable to immune suppressive medications. At the same time, older age is associated with lower antibody and cellular immune response to COVID-19 vaccines.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Chen C-M, Chen H-J, Chen W-S, Lin C-C, Hsu C-C, Hsu Y-H. Clinical effectiveness of influenza vaccination in patients with rheumatoid arthritis. Int J Rheum Dis 2018;21:1246–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

