Oxidation characteristic and thermal runaway of isoprene
- PMID: 37660031
- PMCID: PMC10475201
- DOI: 10.1186/s13065-023-01016-y
Oxidation characteristic and thermal runaway of isoprene
Abstract
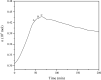
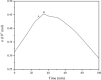
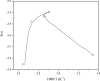
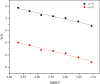
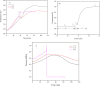
In this study, the oxidation characteristics of isoprene were investigated using a custom-designed mini closed pressure vessel test (MCPVT). The results show that isoprene is unstable and polymerization occurs under a nitrogen atmosphere. Under an oxygen atmosphere, the oxidation process of isoprene was divided into three stages: (1) isoprene reacts with oxygen to produce peroxide; (2) Peroxides produce free radicals through thermal decomposition; (3) Free radicals cause complex oxidation and thermal runaway reactions. The oxidation of isoprene conforms to the second-order reaction kinetics, and the activation energy was 86.88 kJ·mol-1. The thermal decomposition characteristics of the total oxidation product and purified peroxide mixture were determined by differential scanning calorimetry (DSC). The initial exothermic temperatures Ton were 371.17 K and 365.84 K, respectively. And the decomposition heat QDSC were 816.66 J·g-1 and 991.08 J·g-1, respectively. It indicates that high concentration of isoprene peroxide has a high risk of thermal runaway. The results of thermal runaway experiment showed that the temperature and pressure of isoprene oxidation were prone to rise rapidly, which indicates that the oxidation reaction was dangerous. The reaction products of isoprene were analyzed by gas chromatography-mass spectrometry (GC-MS). The main oxidation products were methyl vinyl ketone, methacrolein, 3-methylfuran, etc. The main thermal runaway products were dimethoxymethane, 2,3-pentanedione, naphthalene, etc. Based on the reaction products, the possible reaction pathway of isoprene was proposed.
Keywords: Hazard; Isoprene; Oxidation kinetic; Peroxide; Thermal runaway.
© 2023. Springer Nature Switzerland AG.
Conflict of interest statement
We have no competing interests.
Figures












Similar articles
-
Characteristics and hazards of the cinnamaldehyde oxidation process.RSC Adv. 2020 May 20;10(32):19124-19133. doi: 10.1039/c9ra10820c. eCollection 2020 May 14. RSC Adv. 2020. PMID: 35518288 Free PMC article.
-
Thermal decomposition characteristics of BHT and its peroxide (BHTOOH).BMC Chem. 2024 Apr 29;18(1):87. doi: 10.1186/s13065-024-01190-7. BMC Chem. 2024. PMID: 38685077 Free PMC article.
-
Thermal stability of levopimaric acid and its oxidation products.BMC Chem. 2023 Sep 20;17(1):118. doi: 10.1186/s13065-023-01031-z. BMC Chem. 2023. PMID: 37730608 Free PMC article.
-
Monitoring OH-initiated oxidation kinetics of isoprene and its products using online mass spectrometry.Environ Sci Technol. 2005 Feb 15;39(4):1030-6. doi: 10.1021/es049438f. Environ Sci Technol. 2005. PMID: 15773474
-
Atmospheric aqueous phase radical chemistry of the isoprene oxidation products methacrolein, methyl vinyl ketone, methacrylic acid and acrylic acid--kinetics and product studies.Phys Chem Chem Phys. 2014 Apr 7;16(13):6257-72. doi: 10.1039/c3cp54859g. Phys Chem Chem Phys. 2014. PMID: 24569503
Cited by
-
Accidents involving lithium-ion batteries in non-application stages: incident characteristics, environmental impacts, and response strategies.BMC Chem. 2025 Apr 13;19(1):94. doi: 10.1186/s13065-025-01445-x. BMC Chem. 2025. PMID: 40223134 Free PMC article. Review.
References
-
- Whited GM, Feher FJ, Benko DA, Cervin MA, Sanford KJ. Technology update: Development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Ind Biotechnol. 2010;6(3):152–163. doi: 10.1089/ind.2010.6.152. - DOI
-
- Takabe K, Agata A, Katagiri T, Tanaka J. Direct formation of myrcene from isoprene. Synthesis. 1977;5:307–308. doi: 10.1055/s-1977-24369. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
