Rescue of dendritic cells from glycolysis inhibition improves cancer immunotherapy in mice
- PMID: 37660049
- PMCID: PMC10475105
- DOI: 10.1038/s41467-023-41016-z
Rescue of dendritic cells from glycolysis inhibition improves cancer immunotherapy in mice
Abstract
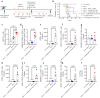
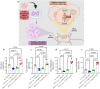
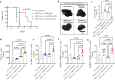
Inhibition of glycolysis in immune cells and cancer cells diminishes their activity, and thus combining immunotherapies with glycolytic inhibitors is challenging. Herein, a strategy is presented where glycolysis is inhibited in cancer cells using PFK15 (inhibitor of PFKFB3, rate-limiting step in glycolysis), while simultaneously glycolysis and function is rescued in DCs by delivery of fructose-1,6-biphosphate (F16BP, one-step downstream of PFKFB3). To demonstrate the feasibility of this strategy, vaccine formulations are generated using calcium-phosphate chemistry, that incorporate F16BP, poly(IC) as adjuvant, and phosphorylated-TRP2 peptide antigen and tested in challenging and established YUMM1.1 tumours in immunocompetent female mice. Furthermore, to test the versatility of this strategy, adoptive DC therapy is developed with formulations that incorporate F16BP, poly(IC) as adjuvant and mRNA derived from B16F10 cells as antigens in established B16F10 tumours in immunocompetent female mice. F16BP vaccine formulations rescue DCs in vitro and in vivo, significantly improve the survival of mice, and generate cytotoxic T cell (Tc) responses by elevating Tc1 and Tc17 cells within the tumour. Overall, these results demonstrate that rescuing glycolysis of DCs using metabolite-based formulations can be utilized to generate immunotherapy even in the presence of glycolytic inhibitor.
© 2023. Springer Nature Limited.
Conflict of interest statement
A.P.A. has some rights reserved for the technology presented. S.I., A.P.S., J.L.M., N.D.N., A.S., C.W., K.L., A.T., T.K., M.H., N.A., M.M.C.S.J., A.E., J.Y., M.C. have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

