Phagotrophic protists preserve antibiotic-resistant opportunistic human pathogens in the vegetable phyllosphere
- PMID: 37660098
- PMCID: PMC10475086
- DOI: 10.1038/s43705-023-00302-z
Phagotrophic protists preserve antibiotic-resistant opportunistic human pathogens in the vegetable phyllosphere
Abstract
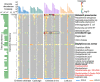
Food safety of leafy greens is an emerging public health issue as they can harbor opportunistic human pathogens (OHPs) and expose OHPs to consumers. Protists are an integral part of phyllosphere microbial ecosystems. However, our understanding of protist-pathogen associations in the phyllosphere and their consequences on public health remains poor. Here, we examined phyllosphere protists, human pathogen marker genes (HPMGs), and protist endosymbionts from four species of leafy greens from major supermarkets in Xiamen, China. Our results showed that Staphylococcus aureus and Klebsiella pneumoniae were the dominant human pathogens in the vegetable phyllosphere. The distribution of HPMGs and protistan communities differed between vegetable species, of which Chinese chive possessed the most diverse protists and highest abundance of HPMGs. HPMGs abundance positively correlated with the diversity and relative abundance of phagotrophic protists. Whole genome sequencing further uncovered that most isolated phyllosphere protists harbored multiple OHPs which carried antibiotic resistance genes, virulence factors, and metal resistance genes and had the potential to HGT. Colpoda were identified as key phagotrophic protists which positively linked to OHPs and carried diverse resistance and virulence potential endosymbiont OHPs including Pseudomonas nitroreducens, Achromobacter xylosoxidans, and Stenotrophomonas maltophilia. We highlight that phyllosphere protists contribute to the transmission of resistant OHPs through internalization and thus pose risks to the food safety of leafy greens and human health. Our study provides insights into the protist-OHP interactions in the phyllosphere, which will help in food safety surveillance and human health.
© 2023. ISME Publications B.V.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ferreira G, Turk Z. An overview of organic vegetable production in the United States. USDA Economic Research Service. 2016;VGS-357-SA2. https://www.ers.usda.gov/webdocs/outlooks/74639/60319_vgs-357-sa2_organi....
-
- Zhou SYD, Zhu D, Giles M, Yang XR, Daniell T, Neilson R, et al. Phyllosphere of staple crops under pig manure fertilization, a reservoir of antibiotic resistance genes. Environ Pollut. 2019;252:227–35. - PubMed
-
- Lenzi A, Marvasi M, Baldi A. Agronomic practices to limit pre- and post-harvest contamination and proliferation of human pathogenic Enterobacteriaceae in vegetable produce. Food Control. 2021;119:107486.
-
- Neil KP, Gwen B, Kathryn MCOJ, Eija T, Carlota M, Musser KA, et al. A novel vehicle for transmission of Escherichia coli O157:H7 to humans: multistate outbreak of E. coli O157:H7 infections associated with consumption of ready-to-bake commercial prepackaged cookie dough-United States, 2009. Clin Infect Dis. 2012;54:511–8. - PubMed
-
- Liu Q, Jin X, Feng X, Yang H, Fu C. Inactivation kinetics of Escherichia coli O157:H7 and Salmonella Typhimurium on organic carrot (Daucus carota L.) treated with low concentration electrolyzed water combined with short-time heat treatment. Food Control. 2019;106:106702.

