Measuring time in buprenorphine treatment stages among people with HIV and opioid use disorder by retention definition and its association with cocaine and hazardous alcohol use
- PMID: 37660116
- PMCID: PMC10474763
- DOI: 10.1186/s13722-023-00408-8
Measuring time in buprenorphine treatment stages among people with HIV and opioid use disorder by retention definition and its association with cocaine and hazardous alcohol use
Abstract
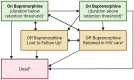
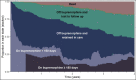
Background: We use a novel, longitudinal approach to describe average time spent in opioid use disorder (OUD) cascade of care stages for people with HIV (PWH) and with OUD, incorporating four definitions of treatment retention. Using this approach, we describe the impact of cocaine or hazardous alcohol use on time spent retained on buprenorphine.
Methods: We followed PWH with OUD enrolled in the Johns Hopkins HIV Clinical Cohort from their first buprenorphine treatment episode between 2013 and 2020. We estimated 4-year restricted mean time spent on buprenorphine below buprenorphine retention threshold, on buprenorphine above retention threshold, off buprenorphine and in HIV care, loss to follow-up, and death. Retention definitions were based on retention threshold (180 vs 90 days) and allowable treatment gap (7 vs 30 days). Differences in 2-year restricted mean time spent retained on buprenorphine were estimated for patients with and without cocaine or hazardous alcohol use.
Results: The study sample (N = 179) was 63% male, 82% non-Hispanic Black, and mean age was 53 (SD 8) years. Patients spent on average 13.9 months (95% CI 11.4, 16.4) on buprenorphine over 4 years. There were differences in time spent retained on buprenorphine based on the retention definition, ranging from 6.5 months (95% CI 4.6, 8.5) to 9.6 months (95% CI 7.4, 11.8). Patients with cocaine use spent fewer months retained on buprenorphine. There were no differences for patients with hazardous alcohol use.
Conclusions: PWH with OUD spend relatively little time receiving buprenorphine in their HIV primary care clinic. Concurrent cocaine use at buprenorphine initiation negatively impact time on buprenorphine.
Keywords: Buprenorphine; Opioid use disorder; Polysubstance use; Retention.
© 2023. Evans Medical Foundation, Inc. and BioMed Central Ltd.
Conflict of interest statement
The authors have no competing interests.
Figures
Similar articles
-
Pain, Substance Use Disorders, Mental Health, and Buprenorphine Treatment among Patients With and Without HIV.AIDS Behav. 2024 Dec;28(12):3994-4004. doi: 10.1007/s10461-024-04494-w. Epub 2024 Sep 12. AIDS Behav. 2024. PMID: 39264485 Free PMC article.
-
Predictors of engagement and retention in care at a low-threshold substance use disorder bridge clinic.J Subst Abuse Treat. 2022 Oct;141:108848. doi: 10.1016/j.jsat.2022.108848. Epub 2022 Jul 29. J Subst Abuse Treat. 2022. PMID: 35926256
-
Polysubstance use and association with opioid use disorder treatment in the US Veterans Health Administration.Addiction. 2021 Jan;116(1):96-104. doi: 10.1111/add.15116. Epub 2020 Jul 7. Addiction. 2021. PMID: 32428386
-
The impact of psychostimulant use on office based buprenorphine treatment retention.Harm Reduct J. 2025 Apr 12;22(1):51. doi: 10.1186/s12954-025-01201-3. Harm Reduct J. 2025. PMID: 40221764 Free PMC article.
-
Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder.JAMA Pediatr. 2018 Nov 1;172(11):1029-1037. doi: 10.1001/jamapediatrics.2018.2143. JAMA Pediatr. 2018. PMID: 30208470 Free PMC article.
References
-
- Blondino CT, Gormley MA, Taylor DDH, Lowery E, Clifford JS, Burkart B, et al. The Influence of Co-occurring substance use on the effectiveness of opiate treatment programs according to intervention type. Epidemiol Rev. 2020;42:57–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

