How to update a living systematic review and keep it alive during a pandemic: a practical guide
- PMID: 37660117
- PMCID: PMC10474670
- DOI: 10.1186/s13643-023-02325-y
How to update a living systematic review and keep it alive during a pandemic: a practical guide
Abstract
Background: The covid-19 pandemic has highlighted the role of living systematic reviews. The speed of evidence generated during the covid-19 pandemic accentuated the challenges of managing high volumes of research literature.
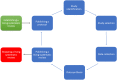
Methods: In this article, we summarise the characteristics of ongoing living systematic reviews on covid-19, and we follow a life cycle approach to describe key steps in a living systematic review.
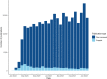
Results: We identified 97 living systematic reviews on covid-19, published up to 7th November 2022, which focused mostly on the effects of pharmacological interventions (n = 46, 47%) or the prevalence of associated conditions or risk factors (n = 30, 31%). The scopes of several reviews overlapped considerably. Most living systematic reviews included both observational and randomised study designs (n = 45, 46%). Only one-third of the reviews has been updated at least once (n = 34, 35%). We address practical aspects of living systematic reviews including how to judge whether to start a living systematic review, methods for study identification and selection, data extraction and evaluation, and give recommendations at each step, drawing from our own experience. We also discuss when it is time to stop and how to publish updates.
Conclusions: Methods to improve the efficiency of searching, study selection, and data extraction using machine learning technologies are being developed, their performance and applicability, particularly for reviews based on observational study designs should improve, and ways of publishing living systematic reviews and their updates will continue to evolve. Finally, knowing when to end a living systematic review is as important as knowing when to start.
Keywords: Covid-19; Epidemiology; Public health; Research design.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
NL declares that her institution has received funding from the Swiss National Science Foundation (176233) and the European Union Horizon 2020 research and innovation programme — project EpiPose (grant agreement number 101003688). The other authors declare that they have no competing interests.
Figures