Exploring the capture and desorption of CO2 on graphene oxide foams supported by computational calculations
- PMID: 37660192
- PMCID: PMC10475065
- DOI: 10.1038/s41598-023-41683-4
Exploring the capture and desorption of CO2 on graphene oxide foams supported by computational calculations
Erratum in
-
Author Correction: Exploring the capture and desorption of CO2 on graphene oxide foams supported by computational calculations.Sci Rep. 2025 Feb 12;15(1):5291. doi: 10.1038/s41598-025-88643-8. Sci Rep. 2025. PMID: 39939700 Free PMC article. No abstract available.
Abstract
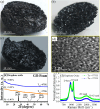
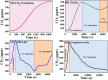
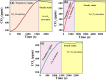
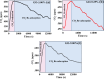
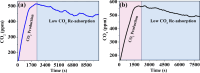
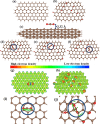
In the last decade, the highest levels of greenhouse gases (GHG) in the atmosphere have been recorded, with carbon dioxide (CO2) being one of the GHGs that most concerns mankind due to the rate at which it is generated on the planet. Given its long time of permanence in the atmosphere (between 100 to 150 years); this has deployed research in the scientific field focused on the absorption and desorption of CO2 in the atmosphere. This work presents the study of CO2 adsorption employing materials based on graphene oxide (GO), such as GO foams with different oxidation percentages (3.00%, 5.25%, and 9.00%) in their structure, obtained via an environmentally friendly method. The characterization of CO2 adsorption was carried out in a closed system, within which were placed the GO foams and other CO2 adsorbent materials (zeolite and silica gel). Through a controlled chemical reaction, production of CO2 was conducted to obtain CO2 concentration curves inside the system and calculate from these the efficiency, obtained between 86.28 and 92.20%, yield between 60.10 and 99.50%, and effectiveness of CO2 adsorption of the materials under study. The results obtained suggest that GO foams are a promising material for carbon capture and the future development of a new clean technology, given their highest CO2 adsorption efficiency and yield.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Bandilla, K. W. Carbon capture and storage. in Future Energy: Improved, Sustainable and Clean Options for Our Planet, 669–692. 10.1016/B978-0-08-102886-5.00031-1 (2020).
-
- Zhang, Z., Borhani, T. N. G. & El-Naas, M. H. Carbon capture. in Exergetic, Energetic and Environmental Dimensions, 997–1016. 10.1016/B978-0-12-813734-5.00056-1 (2018).
-
- Yang, M., Ma, C., Xu, M., Wang, S. & Xu, L. Recent advances in CO2 adsorption from air: A review. Curr. Pollut. Rep.5, 272–293. 10.1007/s40726-019-00128-1 (2019). - DOI
LinkOut - more resources
Full Text Sources

