Heterozygous nonsense variants in the ferritin heavy-chain gene FTH1 cause a neuroferritinopathy
- PMID: 37660254
- PMCID: PMC10510067
- DOI: 10.1016/j.xhgg.2023.100236
Heterozygous nonsense variants in the ferritin heavy-chain gene FTH1 cause a neuroferritinopathy
Abstract
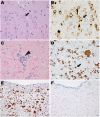
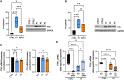
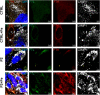
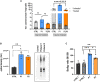
Ferritin, the iron-storage protein, is composed of light- and heavy-chain subunits, encoded by FTL and FTH1, respectively. Heterozygous variants in FTL cause hereditary neuroferritinopathy, a type of neurodegeneration with brain iron accumulation (NBIA). Variants in FTH1 have not been previously associated with neurologic disease. We describe the clinical, neuroimaging, and neuropathology findings of five unrelated pediatric patients with de novo heterozygous FTH1 variants. Children presented with developmental delay, epilepsy, and progressive neurologic decline. Nonsense FTH1 variants were identified using whole-exome sequencing, with a recurrent variant (p.Phe171∗) identified in four unrelated individuals. Neuroimaging revealed diffuse volume loss, features of pontocerebellar hypoplasia, and iron accumulation in the basal ganglia. Neuropathology demonstrated widespread ferritin inclusions in the brain. Patient-derived fibroblasts were assayed for ferritin expression, susceptibility to iron accumulation, and oxidative stress. Variant FTH1 mRNA transcripts escape nonsense-mediated decay (NMD), and fibroblasts show elevated ferritin protein levels, markers of oxidative stress, and increased susceptibility to iron accumulation. C-terminal variants in FTH1 truncate ferritin's E helix, altering the 4-fold symmetric pores of the heteropolymer, and likely diminish iron-storage capacity. FTH1 pathogenic variants appear to act by a dominant, toxic gain-of-function mechanism. The data support the conclusion that truncating variants in the last exon of FTH1 cause a disorder in the spectrum of NBIA. Targeted knockdown of mutant FTH1 transcript with antisense oligonucleotides rescues cellular phenotypes and suggests a potential therapeutic strategy for this pediatric neurodegenerative disorder.
Keywords: anti-sense; dominant negative; exome sequencing; gene disease discovery; pediatric genetics; pediatric neurology.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Update of
-
Heterozygous Nonsense Variants in the Ferritin Heavy Chain Gene FTH1 Cause a Novel Pediatric Neuroferritinopathy.medRxiv [Preprint]. 2023 Jan 31:2023.01.30.23285099. doi: 10.1101/2023.01.30.23285099. medRxiv. 2023. Update in: HGG Adv. 2023 Oct 12;4(4):100236. doi: 10.1016/j.xhgg.2023.100236. PMID: 36778397 Free PMC article. Updated. Preprint.
Similar articles
-
Heterozygous Nonsense Variants in the Ferritin Heavy Chain Gene FTH1 Cause a Novel Pediatric Neuroferritinopathy.medRxiv [Preprint]. 2023 Jan 31:2023.01.30.23285099. doi: 10.1101/2023.01.30.23285099. medRxiv. 2023. Update in: HGG Adv. 2023 Oct 12;4(4):100236. doi: 10.1016/j.xhgg.2023.100236. PMID: 36778397 Free PMC article. Updated. Preprint.
-
Mutant L-chain ferritins that cause neuroferritinopathy alter ferritin functionality and iron permeability.Metallomics. 2019 Oct 16;11(10):1635-1647. doi: 10.1039/c9mt00154a. Metallomics. 2019. PMID: 31513212 Free PMC article.
-
Iron loading-induced aggregation and reduction of iron incorporation in heteropolymeric ferritin containing a mutant light chain that causes neurodegeneration.Biochim Biophys Acta. 2011 Apr;1812(4):544-8. doi: 10.1016/j.bbadis.2010.10.010. Epub 2010 Oct 26. Biochim Biophys Acta. 2011. PMID: 21029774 Free PMC article.
-
Pathogenic mechanism and modeling of neuroferritinopathy.Cell Mol Life Sci. 2021 Apr;78(7):3355-3367. doi: 10.1007/s00018-020-03747-w. Epub 2021 Jan 13. Cell Mol Life Sci. 2021. PMID: 33439270 Free PMC article. Review.
-
Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism.Neurobiol Dis. 2015 Sep;81:134-43. doi: 10.1016/j.nbd.2015.02.007. Epub 2015 Mar 12. Neurobiol Dis. 2015. PMID: 25772441 Free PMC article. Review.
Cited by
-
Whole-exome sequencing identifies protein-coding variants associated with brain iron in 29,828 individuals.Nat Commun. 2024 Jul 2;15(1):5540. doi: 10.1038/s41467-024-49702-2. Nat Commun. 2024. PMID: 38956042 Free PMC article.
-
scaLR: a low-resource deep neural network-based platform for single cell analysis and biomarker discovery.Brief Bioinform. 2025 May 1;26(3):bbaf243. doi: 10.1093/bib/bbaf243. Brief Bioinform. 2025. PMID: 40439670 Free PMC article.
-
Transcriptome Landscape of Cancer-Associated Fibroblasts in Human PDAC.Adv Sci (Weinh). 2025 May;12(20):e2415196. doi: 10.1002/advs.202415196. Epub 2025 Feb 28. Adv Sci (Weinh). 2025. PMID: 40019403 Free PMC article.
-
Neuroferritinopathy Human-Induced Pluripotent Stem Cell-Derived Astrocytes Reveal an Active Role of Free Intracellular Iron in Astrocyte Reactivity.Int J Mol Sci. 2025 Jun 27;26(13):6197. doi: 10.3390/ijms26136197. Int J Mol Sci. 2025. PMID: 40649975 Free PMC article.
-
Dimethyl malonate preserves brain and neurobehavioral phenotype following neonatal hypoxia-ischemia by inhibiting FTH1-mediated ferritinophagy.Redox Biol. 2025 Jul 29;86:103792. doi: 10.1016/j.redox.2025.103792. Online ahead of print. Redox Biol. 2025. PMID: 40749519 Free PMC article.
References
-
- Nakamura T., Naguro I., Ichijo H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 2019;1863:1398–1409. - PubMed
-
- Miller C.J., Rose A.L., Waite T.D. Importance of Iron Complexation for Fenton-Mediated Hydroxyl Radical Production at Circumneutral pH. Front. Mar. Sci. 2016;3
-
- Fisher J., Devraj K., Ingram J., Slagle-Webb B., Madhankumar A.B., Liu X., Klinger M., Simpson I.A., Connor J.R. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am. J. Physiol. Cell Physiol. 2007;293:C641–C649. - PubMed
-
- Connor J.R., Boeshore K.L., Benkovic S.A., Menzies S.L. Isoforms of ferritin have a specific cellular distribution in the brain. J. Neurosci. Res. 1994;37:461–465. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

