Accurate Documentation Contributes to Guideline-concordant Surveillance of Nonmuscle Invasive Bladder Cancer: A Multisite Department of Veterans Affairs Study
- PMID: 37660946
- PMCID: PMC10901298
- DOI: 10.1016/j.urology.2023.08.014
Accurate Documentation Contributes to Guideline-concordant Surveillance of Nonmuscle Invasive Bladder Cancer: A Multisite Department of Veterans Affairs Study
Abstract
Objective: To determine if accurate documentation of bladder cancer risk was associated with a clinician surveillance recommendation that is concordant with AUA guidelines among patients with nonmuscle invasive bladder cancer (NMIBC).
Methods: We prospectively collected data from cystoscopy encounter notes from four Department of Veterans Affairs (VA) sites to ascertain whether they included accurate documentation of bladder cancer risk and a recommendation for a guideline-concordant surveillance interval. Accurate documentation was a clinician-recorded risk classification matching a gold standard assigned by the research team. Clinician recommendations were guideline-concordant if the clinician recorded a surveillance interval that was in line with the AUA guideline.
Results: Among 296 encounters, 75 were for low-, 98 for intermediate-, and 123 for high-risk NMIBC. 52% of encounters had accurate documentation of NMIBC risk. Accurate documentation of risk was less common among encounters for low-risk bladder cancer (36% vs 52% for intermediate- and 62% for high-risk, P < .05). Guideline-concordant surveillance recommendations were also less common in patients with low-risk bladder cancer (67% vs 89% for intermediate- and 94% for high-risk, P < .05). Accurate documentation was associated with a 29% and 15% increase in guideline-concordant surveillance recommendations for low- and intermediate-risk disease, respectively (P < .05).
Conclusion: Accurate risk documentation was associated with more guideline-concordant surveillance recommendations among low- and intermediate-risk patients. Implementation strategies facilitating assessment and documentation of risk may be useful to reduce overuse of surveillance in this group and to prevent unnecessary cost, anxiety, and procedural harms.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest Florian R. Schroeck reports research funding from Pacific Edge, Ltd.
Figures
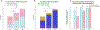
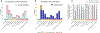
Similar articles
-
Integration and evaluation of implementation strategies to improve guideline-concordant bladder cancer surveillance: a prospective observational study.Implement Sci Commun. 2025 Apr 7;6(1):37. doi: 10.1186/s43058-025-00721-0. Implement Sci Commun. 2025. PMID: 40197353 Free PMC article.
-
The impact of low- versus high-intensity surveillance cystoscopy on surgical care and cancer outcomes in patients with high-risk non-muscle-invasive bladder cancer (NMIBC).PLoS One. 2020 Mar 23;15(3):e0230417. doi: 10.1371/journal.pone.0230417. eCollection 2020. PLoS One. 2020. PMID: 32203532 Free PMC article.
-
Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guérin (BCG): results of an international individual patient data survey (IPDS).BJU Int. 2013 Oct;112(6):742-50. doi: 10.1111/bju.12012. Epub 2013 Mar 1. BJU Int. 2013. PMID: 23452187 Free PMC article. Clinical Trial.
-
Follow-up in non-muscle-invasive bladder cancer-International Bladder Cancer Network recommendations.Urol Oncol. 2016 Oct;34(10):460-8. doi: 10.1016/j.urolonc.2016.05.028. Epub 2016 Jun 29. Urol Oncol. 2016. PMID: 27368880 Review.
-
European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update.Eur Urol. 2019 Nov;76(5):639-657. doi: 10.1016/j.eururo.2019.08.016. Epub 2019 Aug 20. Eur Urol. 2019. PMID: 31443960 Review.
Cited by
-
Integration and evaluation of implementation strategies to improve guideline-concordant bladder cancer surveillance: a prospective observational study.Implement Sci Commun. 2025 Apr 7;6(1):37. doi: 10.1186/s43058-025-00721-0. Implement Sci Commun. 2025. PMID: 40197353 Free PMC article.
References
-
- Van Rhijn BW, Burger M, Lotan Y, et al. 2009. Recurrence and progression of disease in non–muscle-invasive bladder cancer: from epidemiology to treatment strategy. European urology, 56(3), pp.430–442. - PubMed
-
- Chang SS, Boorjian SA, Chou R, et al. 2016. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. The Journal of urology, 196(4), pp.1021–1029. - PubMed
-
- Schroeck FR, Lynch KE, won Chang J, et al. 2018. Extent of risk-aligned surveillance for cancer recurrence among patients with early-stage bladder cancer. JAMA network open, 1(5), pp.e183442–e183442. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2703953 - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

