Thrombotic Microangiopathy after a 15-year Treatment with Interferon Beta-1b in a Patient with Multiple Sclerosis: A Case Report and Review of Literature
- PMID: 37661454
- PMCID: PMC11081907
- DOI: 10.2169/internalmedicine.1846-23
Thrombotic Microangiopathy after a 15-year Treatment with Interferon Beta-1b in a Patient with Multiple Sclerosis: A Case Report and Review of Literature
Abstract
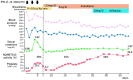
A 54-year-old woman with multiple sclerosis treated with interferon-β (IFN-β)-1b for 15 years presented with sustained hypertension (240/124 mmHg) and retinal bleeding. She had proteinuria, anemia, thrombocytopenia, elevated serum creatinine levels, and haptoglobin depletion. Intravenous nicardipine stabilized her blood pressure, but her renal function and platelet count deteriorated. The initial disintegrin-like metalloprotease with thrombospondin type 1 motifs 13 (ADAMTS13) activity was 28% of normal without its inhibitor. The subsequent peripheral appearance of schistocytes suggested thrombotic microangiopathy (TMA). After IFN-β-1b cessation, the platelet count increased, and the blood pressure stabilized. The ADAMTS13 activity normalized, although the creatinine level did not. TMA may develop after the long-term use of IFN-β without adverse events.
Keywords: disintegrin-like and metalloprotease with thrombospondin type 1 motifs 13 (ADAMTS13); interferon-β (IFN-β); long-term treatment; multiple sclerosis; thrombotic microangiopathy (TMA).
Conflict of interest statement
Figures
Similar articles
-
Thrombotic microangiopathy induced by long-term interferon-β therapy for multiple sclerosis: a case report.Clin Nephrol. 2011 Nov;76(5):396-400. doi: 10.5414/cn106523. Clin Nephrol. 2011. PMID: 22000560
-
Thrombotic microangiopathy caused by interferon β-1b for multiple sclerosis: a case report.CEN Case Rep. 2016 Nov;5(2):179-183. doi: 10.1007/s13730-016-0220-7. Epub 2016 Jun 20. CEN Case Rep. 2016. PMID: 28508977 Free PMC article.
-
Renal thrombotic microangiopathy caused by interferon beta-1a treatment for multiple sclerosis.Drug Des Devel Ther. 2013 Aug 7;7:723-8. doi: 10.2147/DDDT.S42138. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 23950639 Free PMC article.
-
Interferon induced thrombotic microangiopathy (TMA): Analysis and concise review.Crit Rev Oncol Hematol. 2017 Apr;112:103-112. doi: 10.1016/j.critrevonc.2017.02.011. Epub 2017 Feb 17. Crit Rev Oncol Hematol. 2017. PMID: 28325251 Review.
-
Interferon beta-1b-induced postmenopausal bleeding in a patient with multiple sclerosis.Climacteric. 2016 Dec;19(6):599-600. doi: 10.1080/13697137.2016.1244666. Epub 2016 Oct 17. Climacteric. 2016. PMID: 27749097 Review.
Cited by
-
Disease-modifying therapies and hematological disorders: a systematic review of case reports and case series.Front Neurol. 2024 Jun 18;15:1386527. doi: 10.3389/fneur.2024.1386527. eCollection 2024. Front Neurol. 2024. PMID: 38957352 Free PMC article.
-
Thrombotic Microangiopathy as a Life-Threatening Complication of Long-Term Interferon Beta Therapy for Multiple Sclerosis: Clinical Phenotype and Response to Treatment-A Literature Review.J Clin Med. 2024 Mar 11;13(6):1598. doi: 10.3390/jcm13061598. J Clin Med. 2024. PMID: 38541824 Free PMC article. Review.

