MARCH8 downregulation modulates profibrotic responses including myofibroblast differentiation
- PMID: 37661917
- PMCID: PMC10854817
- DOI: 10.1152/ajpcell.00166.2023
MARCH8 downregulation modulates profibrotic responses including myofibroblast differentiation
Abstract
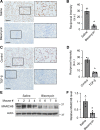
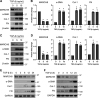
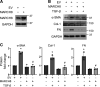
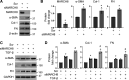
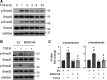
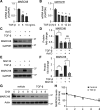
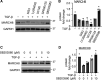
Interstitial lung diseases can result in poor patient outcomes, especially in idiopathic pulmonary fibrosis (IPF), a severe interstitial lung disease with unknown causes. The lack of treatment options requires further understanding of the pathological process/mediators. Membrane-associated RING-CH 8 (MARCH8) has been implicated in immune function regulation and inflammation, however, its role in the development of pulmonary fibrosis and particularly the fibroblast to myofibroblast transition (FMT) remains a gap in existing knowledge. In this study, we demonstrated decreased MARCH8 expression in patients with IPF compared with non-PF controls and in bleomycin-induced PF. TGF-β dose- and time-dependently decreased MARCH8 expression in normal and IPF human lung fibroblast (HLFs), along with induction of FMT markers α-SMA, collagen type I (Col-1), and fibronectin (FN). Interestingly, overexpression of MARCH8 significantly suppressed TGF-β-induced expression of α-SMA, Col-1, and FN. By contrast, the knockdown of MARCH8 using siRNA upregulated basal expression of α-SMA/Col-1/FN. Moreover, MARCH8 knockdown enhanced TGF-β-induced FMT marker expression. These data clearly show that MARCH8 is a critical "brake" for FMT and potentially affects PF. We further found that TGF-β suppressed MARCH8 mRNA expression and the proteasome inhibitor MG132 failed to block MARCH8 decrease induced by TGF-β. Conversely, TGF-β decreases mRNA levels of MARCH8 in a dose- and time-dependent manner, suggesting the transcriptional regulation of MARCH8 by TGF-β. Mechanistically, MARCH8 overexpression suppressed TGF-β-induced Smad2/3 phosphorylation, which may account for the observed effects. Taken together, this study demonstrated an unrecognized role of MARCH8 in negatively regulating FMT and profibrogenic responses relevant to interstitial lung diseases.NEW & NOTEWORTHY MARCH8 is an important modulator of inflammation, immunity, and other cellular processes. We found that MARCH8 expression is downregulated in the lungs of patients with idiopathic pulmonary fibrosis (IPF) and experimental models of pulmonary fibrosis. Furthermore, TGF-β1 decreases MARCH8 transcriptionally in human lung fibroblasts (HLFs). MARCH8 overexpression blunts TGF-β1-induced fibroblast to myofibroblast transition while knockdown of MARCH8 drives this profibrotic change in HLFs. The findings support further exploration of MARCH8 as a novel target in IPF.
Keywords: IPF; MARCH8; TGF-β; fibroblast; lung.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi:10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

