Application of CRISPR-Cas system in the diagnosis and therapy of ESKAPE infections
- PMID: 37662004
- PMCID: PMC10470840
- DOI: 10.3389/fcimb.2023.1223696
Application of CRISPR-Cas system in the diagnosis and therapy of ESKAPE infections
Abstract
Antimicrobial-resistant ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens represent a global threat to human health. ESKAPE pathogens are the most common opportunistic pathogens in nosocomial infections, and a considerable number of their clinical isolates are not susceptible to conventional antimicrobial therapy. Therefore, innovative therapeutic strategies that can effectively deal with ESKAPE pathogens will bring huge social and economic benefits and ease the suffering of tens of thousands of patients. Among these strategies, CRISPR (clustered regularly interspaced short palindromic repeats) system has received extra attention due to its high specificity. Regrettably, there is currently no direct CRISPR-system-based anti-infective treatment. This paper reviews the applications of CRISPR-Cas system in the study of ESKAPE pathogens, aiming to provide directions for the research of ideal new drugs and provide a reference for solving a series of problems caused by multidrug-resistant bacteria (MDR) in the post-antibiotic era. However, most research is still far from clinical application.
Keywords: CRISPR-Cas; ESKAPE; diagnosis; infection; pathogen; therapy.
Copyright © 2023 Qian, Zhou, Li, Zhao, Liu, Yang, Ying and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
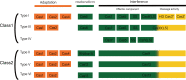
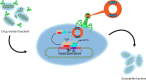
Figures
References
-
- Akinola O. T., Okedigba I., Elutade O. O. (2022). Occurrence, antibiotic susceptibility and resistance genes among Staphylococcus aureus isolated from keypads of automated teller machines (ATM) in a private university, Nigeria. Sci. Afr. 15, 1–8. doi: 10.1016/j.sciaf.2022.e01111 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

