Proton pump inhibitors induced fungal dysbiosis in patients with gastroesophageal reflux disease
- PMID: 37662013
- PMCID: PMC10469693
- DOI: 10.3389/fcimb.2023.1205348
Proton pump inhibitors induced fungal dysbiosis in patients with gastroesophageal reflux disease
Abstract
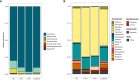
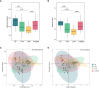
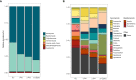
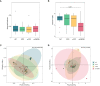
Gut mycobiota inhabits human gastrointestinal lumen and plays a role in human health and disease. We investigated the influence of proton pump inhibitors (PPIs) on gastric mucosal and fecal mycobiota in patients with gastroesophageal reflux diseases (GERD) by using Internal Transcribed Spacer 1 sequencing. A total of 65 participants were included, consisting of the healthy control (HC) group, GERD patients who did not use PPIs (nt-GERD), and GERD patients who used PPIs, which were further divided into short-term (s-PPI) and long-term PPI user (l-PPI) groups based on the duration of PPI use. The alpha diversity and beta diversity of gastric mucosal mycobiota in GERD patients with PPI use were significantly different from HCs, but there were no differences between s-PPI and l-PPI groups. LEfSe analysis identified Candida at the genus level as a biomarker for the s-PPI group when compared to the nt-GERD group. Meanwhile, Candida, Nothojafnea, Rhizodermea, Ambispora, and Saccharicola were more abundant in the l-PPI group than in the nt-GERD group. Furthermore, colonization of Candida in gastric mucosa was significantly increased after PPI treatment. However, there was no significant difference in Candida colonization between patients with endoscopic esophageal mucosal breaks and those without. There were significant differences in the fecal mycobiota composition between HCs and GERD patients regardless whether or not they used PPI. As compared to nt-GERD patient samples, there was a high abundance of Alternaria, Aspergillus, Mycenella, Exserohilum, and Clitopilus in the s-PPI group. In addition, there was a significantly higher abundance of Alternaria, Aspergillus, Podospora, Phallus, and Monographella in the l-PPI group than nt-GERD patients. In conclusion, our study indicates that dysbiosis of mycobiota was presented in GERD patients in both gastric mucosal and fecal mycobiota. PPI treatment may increase the colonization of Candida in the gastric mucosa in GERD patients.
Keywords: Candida; fecal mycobiota; gastric mucosal mycobiota; gastroesophageal reflux disease; proton pump inhibitor.
Copyright © 2023 Shi, Li, Cai, Zhao, Zhao, Sun and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Effects of Proton Pump Inhibitors on the Gastrointestinal Microbiota in Gastroesophageal Reflux Disease.Genomics Proteomics Bioinformatics. 2019 Feb;17(1):52-63. doi: 10.1016/j.gpb.2018.12.004. Epub 2019 Apr 25. Genomics Proteomics Bioinformatics. 2019. PMID: 31028880 Free PMC article.
-
The Influence of Proton Pump Inhibitors on the Fecal Microbiome of Infants with Gastroesophageal Reflux-A Prospective Longitudinal Interventional Study.Front Cell Infect Microbiol. 2017 Oct 11;7:444. doi: 10.3389/fcimb.2017.00444. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29075620 Free PMC article. Clinical Trial.
-
ARE THE PERSISTENT SYMPTOMS TO PROTON PUMP INHIBITOR THERAPY DUE TO REFRACTORY GASTROESOPHAGEAL REFLUX DISEASE OR TO OTHER DISORDERS?Arq Gastroenterol. 2018 Nov;55Suppl 1(Suppl 1):85-91. doi: 10.1590/S0004-2803.201800000-48. Epub 2018 Oct 4. Arq Gastroenterol. 2018. PMID: 30304291 Review.
-
Randomized controlled trial of transoral incisionless fundoplication vs. proton pump inhibitors for treatment of gastroesophageal reflux disease.Am J Gastroenterol. 2015 Apr;110(4):531-42. doi: 10.1038/ajg.2015.28. Epub 2015 Mar 31. Am J Gastroenterol. 2015. PMID: 25823768 Clinical Trial.
-
The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association.Gastroenterology. 2017 Mar;152(4):706-715. doi: 10.1053/j.gastro.2017.01.031. Gastroenterology. 2017. PMID: 28257716 Review.
Cited by
-
Endoscopic anti-reflux mucosal resection for patients with gastroesophageal reflux disease: Clinical efficacy and impact on gut microbiota.World J Gastrointest Surg. 2025 Jun 27;17(6):103336. doi: 10.4240/wjgs.v17.i6.103336. World J Gastrointest Surg. 2025. PMID: 40584513 Free PMC article.
-
Long-term alterations in gut microbiota following mild COVID-19 recovery: bacterial and fungal community shifts.Front Cell Infect Microbiol. 2025 May 26;15:1565887. doi: 10.3389/fcimb.2025.1565887. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40491436 Free PMC article.
-
Causal relationship between gut microbiota and risk of gastroesophageal reflux disease: a genetic correlation and bidirectional Mendelian randomization study.Front Immunol. 2024 Feb 21;15:1327503. doi: 10.3389/fimmu.2024.1327503. eCollection 2024. Front Immunol. 2024. PMID: 38449873 Free PMC article.
-
In Vitro Acid Resistance of Pathogenic Candida Species in Simulated Gastric Fluid.Gastro Hep Adv. 2024 Nov 23;4(4):100591. doi: 10.1016/j.gastha.2024.100591. eCollection 2025. Gastro Hep Adv. 2024. PMID: 39996247 Free PMC article.
-
Microbiota gut-brain axis: implications for pediatric-onset leukodystrophies.Front Nutr. 2024 Jul 12;11:1417981. doi: 10.3389/fnut.2024.1417981. eCollection 2024. Front Nutr. 2024. PMID: 39070252 Free PMC article. Review.
References
-
- Alhazzani W., Alshamsi F., Belley-Cote E., Heels-Ansdell D., Brignardello-Petersen R., Alquraini M., et al. . (2018). Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med. 44, 1–11. doi: 10.1007/s00134-017-5005-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

