Helicobacter pylori infection altered gastric microbiota in patients with chronic gastritis
- PMID: 37662018
- PMCID: PMC10470091
- DOI: 10.3389/fcimb.2023.1221433
Helicobacter pylori infection altered gastric microbiota in patients with chronic gastritis
Abstract
Objective: The present study aims to investigate the effect of Helicobacter pylori (Hp) infection on gastric mucosal microbiota in patients with chronic gastritis.
Methods: Here recruited a population of 193 patients with both chronic gastritis and positive rapid urease, including 124 patients with chronic atrophic gastritis (CAG) and 69 patients with chronic non-atrophic gastritis (nCAG). Immunoblotting was used to detect four serum Hp antibodies (UreA, UreB, VacA and CagA) to determine the types of virulent Hp-I and avirulent Hp-II infections. Gastric microbiota was profiled by 16S rRNA gene V3-V4 region, and R software was used to present the relationship between the microbial characteristics and the type of Hp infection.
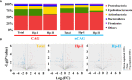
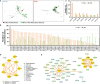
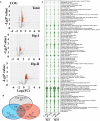
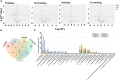
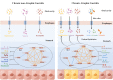
Results: In the stomach of patients with Hp-positive gastritis, the dominant gastric bacterial genera included Ralstonia (23.94%), Helicobacter (20.28%), Pseudonocardia (9.99%), Mesorhizobium (9.21%), Bradyrhizobium (5.05%), and Labrys (4.75%). The proportion of Hp-I infection was significantly higher in CAG patients (91.1%) than in nCAG patients (71.0%) (P < 0.001). The gastric microbiota richness index (observed OTUs, Chao) was significantly lower in CAG patients than in nCAG patients (P <0.05). Compared with avirulent Hp-II infection, virulent Hp-I infection significantly decreased the Shannon index in CAG patients (P <0.05). In nCAG patients, Hp-I infected patients had lower abundances of several dominant gastric bacteria (Aliidiomarina, Reyranella, Halomonas, Pseudomonas, Acidovorax) than Hp-II infected patients. Meanwhile, in CAG patients, Hp-I infected patients occupied lower abundances of several dominant oral bacteria (Neisseria, Staphylococcus and Haemophilus) than Hp-II infected patients. In addition, bile reflux significantly promoted the colonization of dominant oral microbiota (Veillonella, Prevotella 7 and Rothia) in the stomach of CAG patients. There was no significant symbiotic relationship between Helicobacter bacteria and non-Helicobacter bacteria in the stomach of nCAG patients, while Helicobacter bacteria distinctly linked with the non-Helicobacter bacteria (Pseudolabrys, Ralstonia, Bradyrhizobium, Mesorhizobium and Variovorax) in CAG patients.
Conclusions: Virulent Hp infection alters the gastric microbiota, reduces microbial diversity, and enhances the symbiotic relationship between the Helicobacter bacteria and non-Helicobacter bacteria in patients with chronic gastritis. The data provides new evidence for treating Hp infection by improving the gastric microbiota.
Keywords: Helicobacter pylori; chronic gastritis; gastric mucosa; microbiota; symbiotic relationship.
Copyright © 2023 Hua, Xu, Zhu, Xiao, Lu, Wu, Wu, Zhou and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The mutual interactions among Helicobacter pylori, chronic gastritis, and the gut microbiota: a population-based study in Jinjiang, Fujian.Front Microbiol. 2024 Feb 14;15:1365043. doi: 10.3389/fmicb.2024.1365043. eCollection 2024. Front Microbiol. 2024. PMID: 38419635 Free PMC article.
-
Dysregulated gastric microbial communities and functional shifts in chronic atrophic versus non-atrophic gastritis: a Helicobacter pylori-Negative observational study.BMC Gastroenterol. 2025 Apr 29;25(1):304. doi: 10.1186/s12876-025-03900-4. BMC Gastroenterol. 2025. PMID: 40301773 Free PMC article.
-
Does Helicobacter pylori infection affect the structure of bacteria in the gastric mucosa and fluid in patients with chronic antral gastritis?J Gen Appl Microbiol. 2021 Nov 25;67(5):179-185. doi: 10.2323/jgam.2020.08.005. Epub 2021 May 29. J Gen Appl Microbiol. 2021. PMID: 34053980
-
Treatment of Helicobacter pylori infection in atrophic gastritis.World J Gastroenterol. 2018 Jun 14;24(22):2373-2380. doi: 10.3748/wjg.v24.i22.2373. World J Gastroenterol. 2018. PMID: 29904244 Free PMC article. Review.
-
Interactions between Helicobacter pylori and gastroesophageal reflux disease.Esophagus. 2019 Jan;16(1):52-62. doi: 10.1007/s10388-018-0637-5. Epub 2018 Aug 27. Esophagus. 2019. PMID: 30151653 Review.
Cited by
-
Effects of Infection with Different Types of Helicobacter pylori on Gastric Secretion Function: A Cross-Sectional Clinical Study.Int J Gen Med. 2024 Oct 9;17:4539-4549. doi: 10.2147/IJGM.S477480. eCollection 2024. Int J Gen Med. 2024. PMID: 39398484 Free PMC article.
-
Causal associations between Helicobacter Pylori infection and the risk and symptoms of Parkinson's disease: a Mendelian randomization study.Front Immunol. 2024 Aug 6;15:1412157. doi: 10.3389/fimmu.2024.1412157. eCollection 2024. Front Immunol. 2024. PMID: 39165356 Free PMC article.
-
Gastric Cancer and Microbiota: Exploring the Microbiome's Role in Carcinogenesis and Treatment Strategies.Life (Basel). 2025 Jun 23;15(7):999. doi: 10.3390/life15070999. Life (Basel). 2025. PMID: 40724502 Free PMC article. Review.
-
Exploring the genetic associations and causal relationships between antibody responses, immune cells, and various types of breast cancer.Sci Rep. 2024 Nov 19;14(1):28579. doi: 10.1038/s41598-024-79521-w. Sci Rep. 2024. PMID: 39562684 Free PMC article.
-
Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients.Open Life Sci. 2024 Mar 28;19(1):20220839. doi: 10.1515/biol-2022-0839. eCollection 2024. Open Life Sci. 2024. PMID: 38585629 Free PMC article.
References
-
- Atherton J. C., Cao P., Peek J.R. R. M., Tummuru M. K., Blaser M. J., Cover T. L. (1995). Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 30, 17771–17777. doi: 10.1074/jbc.270.30.17771 - DOI - PubMed
-
- Blaser M. J., Perez-Perez G. I., Kleanthous H., Cover T. L., Peek R. M., Chyou P. H., et al. (1995). Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 10, 2111–2115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

