Engineered neurogenesis in naïve adult rat cortex by Ngn2-mediated neuronal reprogramming of resident oligodendrocyte progenitor cells
- PMID: 37662111
- PMCID: PMC10471311
- DOI: 10.3389/fnins.2023.1237176
Engineered neurogenesis in naïve adult rat cortex by Ngn2-mediated neuronal reprogramming of resident oligodendrocyte progenitor cells
Abstract
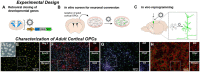
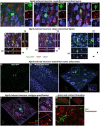
Adult tissue stem cells contribute to tissue homeostasis and repair but the long-lived neurons in the human adult cerebral cortex are not replaced, despite evidence for a limited regenerative response. However, the adult cortex contains a population of proliferating oligodendrocyte progenitor cells (OPCs). We examined the capacity of rat cortical OPCs to be re-specified to a neuronal lineage both in vitro and in vivo. Expressing the developmental transcription factor Neurogenin2 (Ngn2) in OPCs isolated from adult rat cortex resulted in their expression of early neuronal lineage markers and genes while downregulating expression of OPC markers and genes. Ngn2 induced progression through a neuronal lineage to express mature neuronal markers and functional activity as glutamatergic neurons. In vivo retroviral gene delivery of Ngn2 to naive adult rat cortex ensured restricted targeting to proliferating OPCs. Ngn2 expression in OPCs resulted in their lineage re-specification and transition through an immature neuronal morphology into mature pyramidal cortical neurons with spiny dendrites, axons, synaptic contacts, and subtype specification matching local cytoarchitecture. Lineage re-specification of rat cortical OPCs occurred without prior injury, demonstrating these glial progenitor cells need not be put into a reactive state to achieve lineage reprogramming. These results show it may be feasible to precisely engineer additional neurons directly in adult cerebral cortex for experimental study or potentially for therapeutic use to modify dysfunctional or damaged circuitry.
Keywords: NG2 cell; NeuroD1 transcription factor; Neurogenin 2; neural stem/progenitor cells; neuronal replacement; oligodendrocyte precursor cell; reprogramming and differentiation.
Copyright © 2023 Bazarek, Thaqi, King, Mehta, Patel, Briggs, Reisenbigler, Yousey, Miller, Stutzmann, Marr and Peterson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Buffo A., Vosko M. R., Erturk D., Hamann G. F., Jucker M., Rowitch D., et al. . (2005). Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc. Natl. Acad. Sci. U. S. A. 102, 18183–18188. doi: 10.1073/pnas.0506535102, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

